Course Archived Announcements
Please remember to adhere to the University Student Honor Code as we push into final exams and general end-of-semester turmoil. Integrity is so important, please keep this in during your studies and exams. - Dr. McCord

Student Honor Code
As a student of The University of Texas at Austin, I shall abide by the core values* of the University and uphold academic integrity.
* The core values of The University of Texas at Austin are learning, discovery, freedom, leadership, individual opportunity, and responsibility. Each member of the university is expected to uphold these values through integrity, honesty, trust, fairness, and respect toward peers and community.

Final Exam for 11am Class (49335)
Saturday, 5/14, 9am
Room Assignment: ALL students will go to UTC 2.112A for the Final.
NOTICE: There are no preassigned version numbers for the final. The TA and proctors in the room will match you with the appropriate exam copy.
Make sure you bring a calculator, pencil(s), and a UT ID Card (or valid picture ID card).
You want it, you got it. Final Exam Formula Page which is the front page of your exam.
Last Chance Office Hours / Review
Dr. McCord 10am - 12noon, Mon 5/9 in WEL 5.239
Jimmy REVIEW: 3pm - 5pm, Mon 5/9 in WEL 1.308
Kristin 10am - 11am, Tue 5/10 in WEL 2.306 B
Jimmy 12noon - 2pm, Fri 5/13 in WEL 3.140

Final Exam for 10am Class (49330)
Wednesday, 5/11, 9am
Room Assignment: ALL students will go to WEL 2.224 for the Final.
NOTICE: There are no preassigned version numbers for the final. The TA and proctors in the room will match you with the appropriate exam copy.
Make sure you bring a calculator, pencil(s), and a UT ID Card (or valid picture ID card).
An Unfortunate Thing Many of you Neglected...
The syllabus clearly states that you have to have at least a 75% participation rate in order for method 3 to be viable for you. That means you have to have submitted at least 29 quizzes, 24 Reef scores, and at least 3 of the 4 exams. If you do not meet this criteria, then Methods 1 and 2 are the only grading schemes you will have.
DEADLINE is Friday at 8pm!!
Do the eCIS - get +5 points on an Exam!
Pretty easy thing to do for +5 points. Click the headline for the details.
Your NAME and the Completed Surveys table HAVE to be in the screen shot!
337 students have done this and currently have +5 points SHOWING on Canvas for this assignment.
50 students have not submitted anything.
13 student submitted a picture that does NOT meet the criteria for the credit.
Exam 4 is Thursday, 5/5, from 7-9PM
Here is some Stuff you should know for Exam 4.
Here is some Learning Outcomes for Exam 4.
Click below for the 18 question practice exam and key.
Practice Exam 4 | KEY
(note: nothing like #3 will be on our exam)
Version Numbers are HERE ---- deactivated ----
Your VERSION NUMBER determines the ROOM
You can also go to Canvas and check the points for the assignment "Exam 4 version" which matches your version number.
versions 001-199 in UTC 2.112A
versions 200-420 in BUR 106
Early Takers: Those students with documented conflicts and filled out the proper documentation as per the syllabus, will take the exam from 4-6pm in WEL 1.316. SSD students will take the exam at the time and place that your were told via email from the undergraduate office. If you need more help with this please go to WEL 2.212 and ask.
Jimmy's Exam Review Tuesday 5-6pm in CAL 100
Here is download for you: Jimmy's PowerPoint - Exam 4 Review
Dr. McCord's Exam Review Wednesday 2-3pm in WEL 1.316
Dr. McCord's review should once again be recorded and available online afterwards.

Hey - a reminder that the OpenStax site is always a good source to learn more. Here are the direct links.
- Tue, 4/12, 9am
- Thu, 4/14, 9am
- Tue, 4/19, 9am
- Ponder reactions Rates in your head.
- Thu, 4/21, 9am
- Tue, 4/26, 9am
- Fri, 4/29, 11:59pm
Thu, 5/5 Exam 4

Let's TRY and SYNC our REEF scores
This is always fun - you click on button in Canvas, it opens the REEF site, you then login to REEF. You then wait for me to click "sync to canvas" in my REEF app. Then your overall score (percentage of total points) is passed from REEF to Canvas. So much fun.
The real fun is to see if it works or not. After it sort of doesn't work, I call the REEF (or Canvas) people and complain. Fun times all to just get your score over there in Canvas. So I have the links right here to the "assignments" in Canvas that do this.
Pick YOUR Class Below and Follow the Trail to REEF
One last thing - this is not an automated sync. I (Dr. McCord) have to click on a button in order for this to go. However, YOU have to have done the setup above BEFORE I click the button.
Make sure you are up to date on your REEF software
Check your version number and make sure you have REEF R2.4.0 or newer. My iPhone updated itself a day ago and I got version 2.5.0. If you use the web (browser) version of REEF with your laptop, then you don't have to do anything. I plan on using the "target" question type in the future (Friday). This is a way to have you touch the spot on an image that is the answer... like say... where on this pH curve is the equivalence point? or which H in this structure is acidic? Anyway, we'll see if this is a useful feature for kinetics (or not).
Decay directions on Band of Stability Diagram
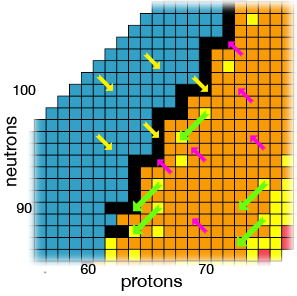
The figure shows a portion (Z between 56 and 77) of the band of stability diagram from our gchem website (same as Wikipedia).
The colored arrows on the diagram represent the transitions for beta decay (yellow), positron emission (pink), and alpha decay (green).
The beta decay and positron emission decays always head directly towards the center black boxes which are the stable nuclides. So beta decay is "one diagonal" towards the stability band.
The alpha decays track back 2 protons and 2 neutrons which is "two diagonals" on the diagram.
Notice how any given "unstable" nuclide will decay to another, and then that to another, and so on. This is what is termed a multiple decay process or "chain". Ultimately, you end up at a stable (black) nuclide on the band of stability.
Exam 3 is Thursday, 4/7, from 7-9PM
Version Numbers are HERE ---- deactivated ----
Your VERSION NUMBER determines the ROOM
You can also go to Canvas and check the points for the assignment "Exam3 Version" which matches your version number.
versions 001-199 in UTC 2.112A
versions 200-420 in BUR 106
Early Takers: Those students with documented conflicts and filled out the proper documentation as per the syllabus, will take the exam from 4-6pm in WEL 1.316. SSD students will take the exam at the time and place that your were told via email from the undergraduate office. If you need more help with this please go to WEL 2.212 and ask.
Jimmy's Exam Review Tuesday 5-6pm in CAL 100
Here is a copy of Jimmy's Electrochemistry Review PowerPoint Presentation .
Dr. McCord's Exam Review Wednesday 2-3pm in WEL 1.316
Dr. McCord's review was recorded and is now available via the "Lecture Recordings" link which is found in the "Class" menu at the top of this webpage. (yes, it IS there)

Hey - a reminder that the OpenStax site is always a good source to learn more. Here is a link to the Electrochemistry Chapter of OpenStax. They have lots of examples worked out and lots of nice diagrams too. It is definitely worth checking out. - Dr. McCord
Here is some Stuff you should know for Exam 3.
Here is some Learning Outcomes for Exam 3.
Here is some Learning Outcomes for Exam 3 - PLUS more.
How do you like my "new and improved" Table of Standard Potentials.
Pick a reduction on the left table, pick an oxidation on the right table and add them: Reduction AND Oxidation Tables Together.
MORE Practice Problems! Below are two homework sets that total 52 questions that you can use for practice as you get ready for Exam 3. They are homework numbers 11 and 12 from my Spring 2013 class. I also included the TTH sections "Model Exam 3" although the cover page still says "Exam 2". This model exam is 25 questions. So wow - you've now got 77 more questions to play with and learn electrochemistry. The keys will go live on Monday next week. TRY and use these like they were an exam - work the whole thing and then grade yourself. You'll get a much better "read" on your level of expertise or lack there of.
- HW11 Electrochemistry I (27 questions) | KEY
Number 23 on HW11 has the RIGHT explanation, but the wrong answer is marked "correct". Here is the correction - HW12 Electrochemistry II (25 questions) | KEY
- Model Exam 3 Spring 2016 (25 questions) | KEY
(note that your cover page will have no formulas)
- Tue, 3/22, 11:59pm
- Thu, 3/24, 9am
- Tue, 3/29, 9am
- Thu, 3/31, 9am
- Tue, 4/5, 9am
Thu, 4/7 Exam 3
Exam 2 Scores Available on Quest
Ouch. I hate to start your Spring Break off with a downer, but the class average was a 66.3 which is disappointing, although not unexpected. I stated over and over the types of questions that would be on the exam and then put them on the exam and many were well below the 50% success rate. Sad, but we will deal with it... Actually, you will deal with it. I will help you out.
Will there be a curve? Yes, but it will once again be an earned curve. We DID have two perfect scores of 100 on the exam, which technically nullifies a straight add-on curve. So what will we do then?
Be Ready to EARN your CURVE when you return from Spring Break.
This means that yet another small quiz will be made available which will have about 6 to 7 questions that were on the exam. So - sometime between now and Monday, 3/21, you'll need to analyze your exam and what you missed, LEARN the exact way to get the answer (look and learn the solution that is shown on the pdf in Quest). The "earn your curve" quiz will go live Monday, 3/21, at 1:30pm and will only be available until 7am the next morning (Tuesday the 22nd). So I WILL be able to talk with you in class about the exam and the curve on that Monday.
Which questions were the worst ones?
Well here is a good starting point on the major missed questions...
- What's the pH at the equivalence point of CH3NH2 titrated with HClO4.
- Which pairs of solutions would result in a buffer?
- Polyprotic acid (H5A) buffer - which Ka do you use?
- Sample of NaHCO3 dissolved into 100 mL. Given two pKa's what is the pH?
- How many acidic protons on this structure?
- What is Kp for A(g)⇌2B(g) given initial A and final total pressure?
- The H+ concentration is 630 times the OH– concentration. What's the pH?
- Add 35.5 mL of KOH to 25.9 mL of HNO3. What is the pH?
All of those questions were at or below the 50% mark. And... every single one of them was discussed and illustrated in class - two of them the very day before the exam.
Here is the link to the Special Exam 2 follow up Quiz on Canvas.

I'll see you back in class on Monday, 3/21, for intro to Electrochemistry! Drive safe, have fun, be free. Peace.
Exam 2 is Thursday, 3/10, from 7-9PM
Version Numbers are HERE ---- deactivated ----
Your VERSION NUMBER determines the ROOM
You can also go to Canvas and check the points for the assignment "Exam2 Version" which matches your version number.
versions 001-210 in UTC 2.112A
versions 211-450 in BUR 106
Early Takers: Those students with documented conflicts and filled out the proper documentation as per the syllabus, will take the exam from 4-6pm in WEL 1.316. SSD students will take the exam at the time and place that your were told via email from the undergraduate office. If you need more help with this please go to WEL 2.212 and ask.
Jimmy's Exam Review Tuesday 5-6pm in CAL 100
Here is a link to Jimmy's Announcement on Canvas about the Review - his PowerPoint is there for you to download and use if you'd like.
Dr. McCord's Exam Review Wednesday 2-3pm in WEL 1.316
Dr. McCord's review was recorded and IS available via the "Lecture Recordings" link which is found in the "Class" menu at the top of this webpage.
WHERE? HOW?... I can't find it... Well it can be a challenge to find the video if you don't search thoroughly. There are "folders" under my name (Dr. McCord) that are Fall 2015, Spring 2016, and Review Session. The Reviews are supposed to land in "Review Session". However, they might not... so search in the "Dr. McCord" folder by date (3/9/2016) and you'll see it... until they move it into the review session folder, then you'll just see it there. Bottom line on this site is to search via date range in each folder except the Fall 2015 one.
My notes (doodles) from the review are on the doodles page.
Preparation...
Get familiar with the Exam 2 Learning Outcomes.
Here are the same Exam 2 Learning Outcomes PLUS what you should be thinking about when you read the outcome.
And something else I've worked on for you. Here is A Bunch of
Stuff you should KNOW about Chemical Equilibria
Stuff you should KNOW about Acid/Base Equilibria
We say "the salt of" a lot in this section. Know why. Read all about it in the SALT of....
Do better, learn more... visit and USE Dr. McCord's Acid/Base Training Page
Once upon a time...(find 118 extra fun problems here)
Reminder: You are MEMORIZING all information now for the exam which includes any necessary formulas. Make this a part of your studies.
You don't have to memorize all the different values for constants and other data values. We will provide you with the data. You just need to know what to do with it.
ACTUALLY, you DO have to know/memorize/use the value for Kw. It is 1.0 × 10-14.
Make Chemistry a DAILY Routine
- Tue, 2/16, 9am
- Thu, 2/18, 9am
- Tue, 2/23, 9am
- Thu, 2/25, 9am
- Tue, 3/1, 9am
Thu, 3/3, 9amFri 3/4 5pm- LE24 - Protonated States
LE25 - Complex Equilibriaremoved
- Tue, 3/8, 9am
Exam 1 - Quiz for a Curve
How to get bonus points (a curve) on Exam 1: Make sure you are well versed (study up again) on sign convention for ∆H and ∆S, how vapor pressure, altitude, and boiling points all work, and how to calculate ∆Hsolution when given the data for it. ALL of those topics were on Exam 1 and came in under 50% for the class. Those are on the Quiz on Canvas.
Here is the Special Exam 1 Follow Up Quiz on Canvas. It is currently worth an additional 4 points on exam 1.
Dr. McCord's very own "Review" for Exam 1
Wednesday, 2/10, 2-3pm in WEL 1.316
The office hour right after class is cancelled - but to replace it, I booked a classroom and will have Office Hours / Review for Exam 1. Room is big so feel free to bring a friend.
Exam 1 is Thursday, 2/11, from 7-9PM
Version Numbers are HERE --- deactivated ---
Your VERSION NUMBER determines the ROOM
You can also go to Canvas and check the points for the assignment "Exam1 Version" which matches your version number.
versions 001-220 in UTC 2.112A
versions 221-450 in BUR 106
Early Takers: Those students with documented conflicts and filled out the proper documentation as per the syllabus, will take the exam from 4-6pm in WEL 1.316. SSD students will take the exam at the time and place that your were told via email from the undergraduate office. If you need more help with this please go to WEL 2.212 and ask.
Preparing for Exam 1
About the Exam: The MWF sections of CH302 will not use "question types" for the exam. Our exams could very well go past the 25 question norm from last semester. Questions will also tend to have varying point totals.
Prep Page. This help page is still evolving, but here it is... Preparing for Exam 1 It is mostly a list of formulas with lots of labeling and annotations from Dr. McCord.
Formulas on the Exam? Yes! And here is a preview copy of the Exam 1 Cover Page with Formulas (v4). (Now with the corrected C-C equation and Henry's Law as well)
What to know? Use this handy listing of Learning Outcomes for Exam 1.
Here are three practice exams (versions 003, 004, and 005). All three are the same exam but randomized in different ways which is just like the real exam. So if you want to retake the exam but with a different order, then try a different version number. Keys to each of these will be activated next week.
practice Exam 1 v003 | KEY practice Exam 1 v004 | KEY practice Exam 1 v005 | KEY
Laude/Vanden Bout Video Review
And... if you're up for it, here is the link to the Laude/Vandenbout ECHO page with their Exam 1 video review. It is called "Exam Review 1 - Full Resolution" from 2/5/2016 at 9am. Remeber to login with your uteid and password.
- Tue, 1/26, 9am
- Thu, 1/28, 9am
- Tue, 2/2, 9am
- Thu, 2/4, 9am
- Tue, 2/9, 9am
Check out yet another example problem of getting the Ksp from solubility of cadmium iodate. I set this up at the end of class on Wednesday. It is "pretty" - have a look.
Calculating MWt via Osmotic Pressure
I worked a problem in class today (Monday, 2/1) where I calculated the molar mass of a substance via the measurement of a colligative property. Think you got it? Try this one:
A solution containing 27.55 mg of an unknown protein per 25.0 mL solution has an osmotic pressure of 3.22 torr at 25 °C. What is the molar mass of the protein?
Get an answer? Here is my very neat solution to the problem to check your answer.
Look! A bonus video! Here is a short video on how to solve a simple dissolved salt freezing point depression problem. It is on YouTube and should be viewed in portrait orientation on your phone. Check it out HERE.
Did you Register with the SSD Office?
Did you register and get an accommodation letter from the SSD office for testing? If the answer is yes, then you need to complete the task in order to get those accommodations. The letter alone is not enough.
As clearly stated in the syllabus, you need to ALSO REGISTER through our chemistry department testing portal. Click the link below and then click to register for SSD accommodations.
Chemistry Department Testing Services
AND... if you have any UT Class related conflict with exam times, you go to the same link and click the link for an alternate time (4-6pm).
WOW! 94% of you have Signed up for REEF!
94% is great, but 100% would be even better. Lets get that number to 100.
Here are the Instructions for REEF Sign Up.
Remember, we are NOT using iClickers. Sign up and BYOD (bring your own device) for in-class response.
Dr. McCord's CH302 for Spring 2016
Yes, you have found my website for my Spring 2016 CH302 class. It is mostly ready to go.
Hang on!
You know who you are... you're thinking about "doing" something - like a quiz or something. Or maybe sign up for Top Hat or REEF or Sapling or some other service. Well just STOP. Just come to class and I will TELL you wonderful amounts of information.
The syllabus is 95% complete but needs a few more tweaks about certain things.
You REALLY want to "get ahead"?
Best thing to do then is start reading the Physical Equilibria chapter (6) on the gchem site. Yeah, use that menu up there and look under sites and find gchem. Go there and read, browse, do whatever.
Did I mention coming to class? I suppose you could follow me on Twitter - that is something you could do (over in the righthand margin).