Chemical Equilibria (general)
the Mass Action Expression (activities)
The mass action expression is an equation that shows the ratio of the product activities raised to the power of their coefficients to that of the reactant activities raised to the power of their coefficients. The expression itself is very easy to correctly write as long as you have a balanced chemical equation to reference. So let's consider the following generic reaction in aqueous solution. Each of the lowercase letters represents the coefficient for that species.
\[a\,{\rm A(aq)} + b\,{\rm B(aq)} \rightleftharpoons c\,{\rm C(aq)} + d\,{\rm D(aq)}\]
the true mass action expression : \( \displaystyle{ {a_{\rm C}^c \cdot a_{\rm D}^d} \over {a_{\rm A}^a \cdot a_{\rm B}^b} }\)
Of course activities can be approximated by using the numeric values of the concentrations (M) for solutions species, and the pressures (atm) for gas species.
Mass Action Expression (concentrations)
We now rewrite the mass action expression for solution species - specifically aqueous species. We give ourselves permission to substitute molar concentration for activity here. This is really just an approximation of the activity version but it works well for dilute solutions.
\[a\,{\rm A(aq)} + b\,{\rm B(aq)} \rightleftharpoons c\,{\rm C(aq)} + d\,{\rm D(aq)}\]
mass action expression : \(\displaystyle{[{\rm C}]^c[{\rm D}]^d\over[{\rm A}]^a[{\rm B}]^b}\)
Square brackets, [ ], around a species always represents the molar concentration (mol/L) of that species in solution.
This equation is easily written in a formalized way - just put all the product concentrations in the numerator (on top) and all the reactant concentrations in the demoninator (on bottom). The coefficients all become powers to the associated concentration term.
NEWS FLASH!
Pure solids and pure liquids have no meaningful concentration term. Their activities are always exactly 1 no matter how much or how little there is. This means that they are NEVER part of a mass action expression or equilibrium expression. LEAVE THEM OUT!!
Concentrations only make sense for dissolved species (think "aqueous" phase) or for gases, pressures make sense.
Mass Action Expression (pressures)
The mass action expression can also be expressed with pressures instead of concentrations. This is of course the usual way of doing things for gaseous reactants and products. Consider once again the generic reaction like before except this time all the species are gases.
\[a\,{\rm A(g)} + b\,{\rm B(g)} \rightleftharpoons c\,{\rm C(g)} + d\,{\rm D(g)}\]
mass action expression : \(\displaystyle{P_{\rm C}^{\,c}P_{\rm D}^{\,d}\over P_{\rm A}^{\,a}P_{\rm B}^{\,b}}\)
Same idea as with the concentration version except now all the terms are partial pressures of each of the species.
Mass Action Expression (mixed terms)
There ARE reactions in this world that have both solution species AND gas species. So which do we use? Concentration or pressure? The answer is that true activities are just numbers, and this means you can mix the two terms. In these cases, the mass action expression has a set of mixed terms - both concentrations (solution species) and pressures (gas species). Here is a real example from electrochemistry where hydrogen ions are reduced to hydrogen gas. This is a half-reaction, which is why electrons are included in the reaction but not the mass action expression.
\[2{\rm H^+(aq)} + 2e^- \rightleftharpoons {\rm H_2(g)}\]
mass action expression : \(\displaystyle{P_{\rm H_2}\over [{\rm H^+}]^2}\)
Notice that partial pressure is used for the gas and concentration is used for the solution species.
Reaction Quotient, Q
The reaction quotient is symbolized with the capital letter Q. Q is simply the numerical value for the mass action expression under any stated set of conditions (concentrations and/or pressures). So Q can essentially be any number between \(0\) and \(\infty\). There is no such thing as a negative concentration or pressure which is why you cannot have negative values of Q.
Take all the concentrations and pressures of all the species in the system and put them into the mass action expression. Then calculate the number - that is Q! It will range from infinitely small (zero, or approaching zero) OR infinitely large (approaching \(\infty\)). Students should THINK of the pivot point within this range as a 1 (one). Generally, when the mass action express calculates to greater than one, then this is a situation where the system is more product favored. Values smaller than one generally mean that the system is more reactant favored.
The value of Q is what is important because you will use that value to determine in which way the system will respond so as to reach the equilibrium state.
IMPORTANT! Q can be all possible values
Q can be every possible value between zero and infinity because you can pick any value you want to go into the mass action expression. You will usually pick the real concentrations and/or pressures that are given in a problem though.
Q is a single number that represents
the current state of the system.
(yes, it IS a state function)
the equilibrium constant, K
The equilibrium constant is symbolized with the capital letter K. Unlike Q, K is a very specific value for a specific reaction under specific conditions. K is a recasting of ΔG° for the reaction.
K is also equal to the mass action expression just like Q, except that all the concentrations and pressures must be their equilibrium values! So think of K as a subset of Q at which equilibrium occurs. There will be lots and lots of possible equilibrium values for a given reaction - just like there are lots of solutions to calculating various points that lie on a straight line. If you know the formula for the straight line (\(y=mx+b\)), then you can generate an infinite number of matched (x,y) coordinates that fall on that line.
A given reaction under specific conditions (lets say standard conditions for reference purposes) will have a very specific value for \(\Delta G^\circ_{\rm rxn}\). The magnitude and the sign of that standard free energy change tells us about the reaction being either strongly or weakly (big kJ or little kJ) spontaneous or non-spontaneous (positive or negative) as written. The equilibrium constant is a recasting of standard free energy. And by recasting I mean that there is an equation that relates the two values. What is that equation?
\[\Delta G^\circ = -RT\ln K\]
now flip that equation
\[K = e^{-\Delta G^\circ\over RT}\]
So the secret is out... K is just a mathematical conversion of ΔG°.
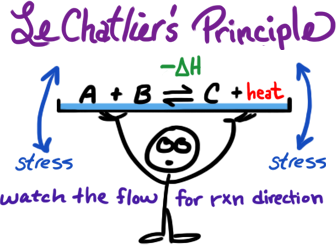 * example shown is for exothermic only
* example shown is for exothermic only
K is where you are going.
Q is where you are at.
Know the way you are heading
from the state that your system is at.
if Q < K shift right →
if Q > K ← shift left
if Q = K no change ⇌