Final Exam Formula Sheet
Click on the big blue button for a preview copy of the formula sheet for the final exam.
And... if you missed it and want to check it out. Here is the Link to our Lecture Recordings where you will find all the recordings of the lectures and more importantly, SHAWN's REVIEW is at the top of the list.
12/4/2013
10:08 am
FINAL EXAM WEEK
Monday and Tuesday 12/9 and 12/10 - "Dead Days" which means no classes at UT.
Wednesday through Tuesday 12/11-17 Final Exams for UT. You HAVE to go to your assigned class for your Final Exam. No exceptions.
CHECK for your Room Assignments on Quest
I know that each class is split into two rooms and both rooms are here in Welch.
Dead Day - Office Hours - Review
- Darren
- Mon 12/9 8-9am WEL 4.238
- Dr. McCord
- Mon 12/9 10-12n WEL 5.239
- Shawn*
- Mon 12/9 5-7pm WEL 2.224 (review)
- Dean
- Tue 12/10 10-12n WEL 2.306 B
- Charles
- Tue 12/10 6-8pm WEL 2.306 A
* We have received a confirmation that Shawn's Review from 5-7pm WILL be recorded and available via the McCord Videos Link - Under class menu, select doodles/video and then click the video link.
Another Exam 4 Correction...
I have decided to grant FULL CREDIT to those students who did the mothball question and used H2O (g) as one of the products. So both -5161 kJ/mol and -4985 kJ/mol are now counted as CORRECT. Check and see your score - the fix has already been made. - Dr. McCord
Exam 4 - FIXED!
UPDATE: (10:35 am Fri 12/6) - The Quest team has finally gone in and FIXED all the Exam 4 scores for our classes. Your current SCORE should now be correct - with the two adjustments that are mentioned below. Please note that your pdf copy available on Quest still shows the wrong choice as correct so please make adjustments in your studying for the final exam.
COME TO CLASS ON FRIDAY! - Learn more details on this matter and more about the final exam. - Dr. McCord
The TWO Questions that were FIXED...
The first question is the bomb calorimeter question about a new snack food. The question asked for the calorie content. The CORRECT answer is 200 Cal/serving. All other choices are wrong and you get zero credit for them.
The second question is the one about tertiary butyl alcohol reacting with hydrobromic acid. You had to use the bond energies table from the front page. The CORRECT answer is -24 kJ/mol. All other choices are incorrect and you get zero points for them.
11/25/2013
10:40 am
Course Instructor Surveys
Course Instructor Surveys are ONLINE for this course.
Please, please, please take the small amount of time to fill out the online survey for this course. I really DO appreciate your cooperation in this matter. Having as many students as possible participate is by far the best case scenario for these surveys. Thanks you so much for participating. - Dr. McCord
Here is the Link to the eCIS page. The survey is available up through the last class day which is Friday, 12/6/13.
No Class on Wednesday
Have a Happy Thanksgiving. See you back in class on Monday, 12/2. Study some thermodynamics when you get the chance. - Dr. McCord
-- no office hours till Monday --
11/13/2013
8:53am
New Quest Assignment

This week a new assignment will appear for your on Quest. It is not part of your grade. But it is very important that you complete it.
Beginning Friday, November 15, you will receive an email from the College of Natural Sciences asking you to complete a short on-line assessment. The data from the assessment will be used to gather data for UT's accreditation process. The assessment consists of 6 short multiple choice questions covering some of the concepts that we have covered or discussed in class. It should only take about 10 minutes to complete.
You will not receive a grade for this assessment. The purpose is to gather data on how well students in the core courses are learning important course concepts. The data for the entire class will be pooled, and the overall performance will be reported to SACS (Southern Association of Colleges and Schools).
The College of Natural Sciences greatly appreciates your participation. Please take this opportunity to test your own knowledge and assist us with this important responsibility.
11/06/2013
8:18pm
iClicker Score Update 11-6

Your iClicker score as of Wednesday 11/6 is now up on Quest. Your score is shown as a percentage of points out of 25 class days (250 points possible for a 100 percent). Scores are available to students that registered their clicker number with iclicker.com as of 8pm Wednesday night (11/6/13). If you still show a zero, it is because you still haven't correctly registered on iclicker.com. You must register on iclicker.com in order to receive any credit for your iclicker responses. You are now officially running out of time to get yourself registered correctly. There are only about 3 students per class that have failed to due this. Thank you to all the students that have correctly done this.
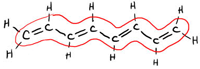
Here's an Old but Useful Lecture Doodle...
The following lecture doodle is from 2010 but has some nice info about Molecular Orbitals and delocalized bonding (conjugated π-systems). Have a look. There are some nice pictures near the end showing the delocalization of electrons in the π-orbitals of nitrate ion, benzene, and a straight-chain conjugated system with 8 sp2 hybridized carbons.
Q-Drop Deadline for the Course is Tuesday 11/5

If you are thinking about dropping the course (and saving your GPA), then you will need to ACT on it this week or by Monday next week. You unfortunately do NOT have the luxury of waiting to see what your score is on Exam 3. By the time you know your exam score, the deadline will have passed and you are in the course for the long haul and for the letter grade that WILL affect your GPA. PLAN AHEAD ON THIS.
UPDATE: Go to the office of the Dean for your major's College and they will initiate and finalize the drop. I don't have to sign for your Q-drop on this first deadline. - Dr. McCord
10/16/2013
11:32am
Maybe you should buy a TEXTBOOK...
Most students will benefit from having an actual textbook for reference purposes and more in depth study. All textbooks offer more coverage of the topics, nice overviews of chapters, and a good set of problems to try at the end of each chapter. Remember that getting a textbook will only help you if you use the textbook. You will need to read it over and over in order to gain a better understanding of the concepts we teach. There are dozens of chemistry textbooks on the market. You should search the used market to find the most cost effective solution for you. Many of these types of books are in our chemistry library here in Welch. A good idea might be to go there first and look through some different texts and authors and then decide what fits you. We do have some basic recommendations below.

FIRST: Our first recommendation for a textbook is Steven Zumdahl's Chemical Principles which is currently up to it's 7th edition which has added a second author (DeCoste). Most students should probably seek out the 5th or 6th editions of this book for a much more cost effective solution (the picture to the left is the 6th edition). Zumdahl is the sole author of those two editions (5th and 6th). He is also the author of quite a few more titles as well. However, students should go for the Chemical Principles title and not the others.

SECOND: We also like the Peter Atkins and Loretta Jones textbook Chemical Principles : The Quest for Insight which is now up to its 6th edition, which like Zumdahl above has added a 3rd author (Laverman) to the line up. Once again, go for the earlier editions - in particular the 4th and 5th editions (5th is shown here). This book is written at a slightly higher level than the Zumdahl book, so if that scares you, go with the Zumdahl book. But this book is really a good source for all the topics we cover and all students should try and get to this level in their reading. You decide.

THIRD: This textbooks has been around for decades... originally by Whitten and Gailey way way back. Then by Whitten, Davis, and Peck... and Stanley... and was titled simply General Chemistry all the way through the 7th edition (the one shown). The title then changed to just plain Chemistry at the 8th edition. They are now currently on the 10th edition of the book. I've had students tell me that this book "speaks to them". I do think this book is a student friendly book also. You decide... the 5th and 6th editions are almost free if you can find them. I also know that there are versions of this textbook in our chemistry library.
10/8/2013
10:25am
iClicker Score Update 10-7

Your iClicker score as of Monday 10/7 is now up on Quest. Your score is shown as a percentage of points out of 14 class days (140 points possible for a 100 percent). Scores are available to students that registered their clicker number with iclicker.com as of 9pm Monday night (10/7/13). If you still show a zero, it is because you still haven't correctly registered on iclicker.com. You must register on iclicker.com in order to receive any credit for your iclicker responses.
10/2/2013
9:30 am
Exam 2 is Tuesday, 10/8, from 7-9 PM

Exam 2 covers ALL of Unit 2 in the eBook and will match nicely with HW04, HW05, and HW06. This also means that the material covered on LMs 12-19 is covered on the exam. You also need to know nomenclature of ionic compounds which includes polyatomic ions. You also need to know the nomenclature of simple binary covalent compounds.
Version Number: Check Quest for your version number for your exam - there will be an assignment named that (like on exam 1) and the "score" or "grade" is your version number. If Quest is being bad, you can always get your version number from a listing posted on the exam room doors.
The exam will be at least 34+1 questions. The +1 is the "extra credit" question just like on exam 1.
Room Assignments: Room assignments for Exam 2 are the same as for exam 1 and are now showing on Quest as an announcement for the class. Remember that the version numbers for the exam (and therefore where you will sit for the exam) will not be posted until Monday morning.
Early Takers: If you signed up for the 4-6 pm time slot, your exam room is different from exam 1 and is now in UTC 4.110.
If you haven't already read it, please read about Exam Day Information which is straight from our eBook FAQ page.
Photoelectric Effect - Simulator
There is a cool Photoelectric Effect Simulator available online at the PHET Simulator for Photoelectric Effect brought to you by the Univ of Colorado in Boulder. Go there and download the Java file - then run it. You can play with lots of variables and see the results. - Dr. McCord
9/23/2013
10:28am
Lunch with Dr. McCord and other faculty
It's "lunch with faculty" on Tuesday this week at the Jester City Limits. Dr. McCord, Dr. LaBrake, and Dr. Vanden Bout will be having lunch from 1:30-2:30 pm at the Jester City Limits.
Please show up if you can. We talk about ANYTHING you want and not just chemistry. We are starting to feel lonely. There is nothing more exciting than having lunch with your teacher. - Dr. McCord
ps - I'll be the one wearing the brown Buc-ee's T-shirt.
iClicker scores are on Quest
Valid through 9/16, check to see that you have a score - if you do, then you registered correctly. If you see a zero and you use an iclicker in class, you did not register correctly and you should try again.
9/2/2013
11:15am
FYI - Entering Numbers on Quest

I had a problem on Quest and I calculated the answer to be 355 mL. How should I enter that into Quest? Hmmmm let me give you WAY TOO MANY examples of all the ways that would be counted as CORRECT in Quest:
355 354.2 351.89210 357 358.1900001 3.55e2 3551329485e-9 0.00003541e7 356. 357 353 352 (any number between 351.45 and 358.55 will be CORRECT)
Things to NOTICE about the numbers: There are NO spaces or other characters in them. ALL of them are within 1% of the "correct" calculated value of 355. There is nothing right or wrong about whether or not you use scientific notation. However, two of my sci not numbers look really silly and aren't really very "human" friendly. Quest isn't human and doesn't care though.
Hope this was educational. - Dr. McCord
9/01/2013
3:35pm
How to ALLOW Content in FireFox and Chrome
 In FireFox and Chrome LOOK for a shield icon (much like that shown here) in the address bar. Click it and ALLOW the content to load - it will fix ALL the problems with seeing videos and the simulator. It is to the LEFT in FireFox but to the far right in Chrome. Eventually, we will get the references fixed and this will resolve itself.
In FireFox and Chrome LOOK for a shield icon (much like that shown here) in the address bar. Click it and ALLOW the content to load - it will fix ALL the problems with seeing videos and the simulator. It is to the LEFT in FireFox but to the far right in Chrome. Eventually, we will get the references fixed and this will resolve itself.
All seems to be well on this issue now. All students should be seeing things correctly on Quest now. You might have to reload the module you are on but the slides should all be showing now.
8/30/2013
10:35am
Pecking ORDER for getting HELP
I (Dr. McCord) am NOT your first line of attack when you need help outside the classroom. There is a certain pecking order so to speak in what you should do for help when outside of the classroom. Please try to follow the following guide lines about getting help.
- Piazza : This is ALWAYS your FIRST line of attack. There are over 800 students doing the same thing - it is very likely that your question has already been asked and answered on piazza. If not, then click new post and ask your question - you'll have over 800 possible "experts" that can start to answer your question.
- Email your Class TA: Next up is to email the class TA with your question. The TA's ARE paid and this IS their job. So I (Dr. McCord) would like for you to first try the TA with your question. They DO know chemistry and are very willing to help. However, remember that many other students could actually BENEFIT from your question being posted on Piazza - so think about the Piazza option again.
- Email Dr. McCord: If the question is such that no student or TA can answer it, then the email will have to come to me (Dr. McCord). READ the FAQ page on the eBook site under "how to send an email to your professor". Follow the rules of etiquette and send the email. I'll will answer the email IF the question hasn't already been answered on the website or on piazza. If it is answered there you will either get no email or a very short one telling you the answer is out there.
8/30/2013
11:25am
Your To Do List
Yes! Go BUY or obtain an iClicker2 for this class. And go Register it on iClicker.com.
Check out Piazza. Ask a Question / Answer a Question.
Do BROWSE our eBook and be READING the first section of Unit 1.
8/29/2013
8:45pm
FUN! Entering Numbers on Quest

This IS in the Student FAQ under the Help menu in Quest - which I said in class to READ. None-the-less, here it is "in your face" on our web page.
So let's say your answer on your calculator is 45.67930234 and it is mL. What do you enter on Quest? If it were me, I'd enter: 45.7 into the Quest input box and then click the "submit" button. You really only need 3 significant figures. Let's do another one...
I calculate some big-ass number where Avogadro's number (NA) is in there. My calculator is telling me that the answer is 3.6793 × 1025 atoms of carbon. So what do I enter into the Quest input box? I enter: 3.68e25 and then click the "submit" button. Do NOT try to enter units - Quest already knows the units because it tells you what they are. Also, do NOT put spaces into your numeric answer, Quest will STOP reading at the very first space.
09/06/2013
10:10am
Heres an Info Box
This is just a test of an info box.