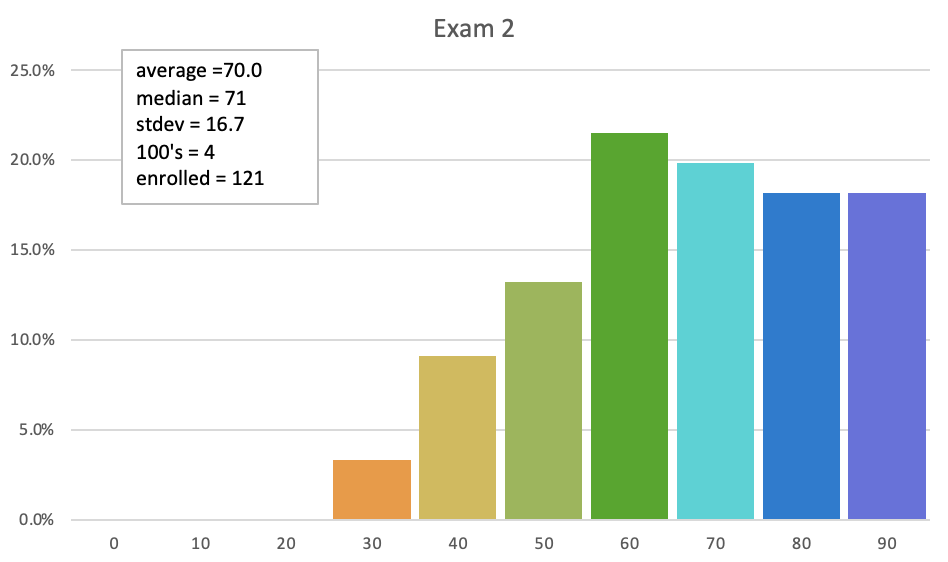
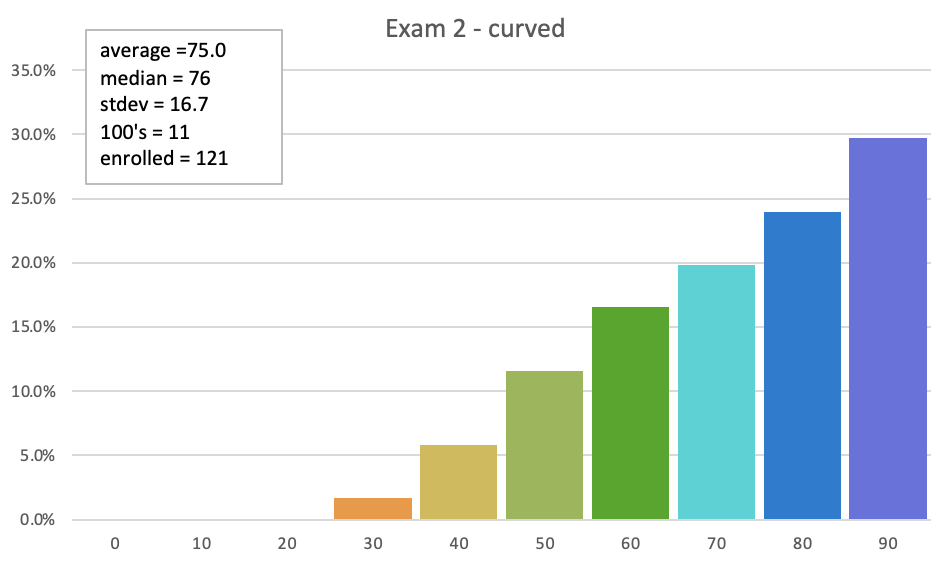
Exam 2
Tuesday 7/27
Exam protocol All you need to know about HOW to take our online exams. Learning Outcomes for Acid/Base Equilibria
Students will be able to...
Understand the strength of an acid (or base) as determined by the percent of ionization in solution.
Identify strong and weak acids and bases.
Identify acid/base conjugate pairs and their relative strengths.
Understand the process of auto-ionization of water and what is meant by acidic, basic, and neutral.
Know the value of K w at 25°C, and the relationship between K a and K b for a conjugate pair.
Convert between hydronium ion concentration, hydroxide ion concentration, pH and pOH for a given solution.
Determine the pH of a strong acid or base solution.
Determine the pH of a weak acid or weak base solution.
Determine the pH of the solution made from the salt of a weak acid or the salt of a weak base.
Recognize and predict the components of a buffer solution.
Calculate the pH of a buffer solution, and a buffer solution after the addition of strong acid or strong base.
Balance a reaction for the neutralization of an acid or base and calculate stoichiometric quantities throughout the reaction (titration).
Determine the majority species for acid/base solutions as well as the pH following neutralization.
Interpret a titration curve plot including calculating the concentration and K a or K b for the analyte.
Understand the concept of an acid/base indicator, and determine which indicators are appropriate for a given titration.
Determine the protonation state (or overall charge) for a polyprotic species at a particular pH.
Apply concepts from equilibria to acid/base problems
acid / base theory
(Lowry-Bronsted definition)
acid = a proton donor
base = a proton acceptor
Dr. McCord'sAcid/Base Trainer Page
buffer = a solution that resists pH change
water
K w = [H+ ][OH- ]
pH = -log[H+ ]
[H+ ] = 10-pH
pOH = -log[OH- ]
[OH- ] = 10-pOH
weak acids / weak bases
acid reaction: + (aq) + A- (aq)
K a =
[H+ ][A- ] [HA]
base reaction: (+ H2 O) ⇌ OH- (aq) + BH+ (aq)
K b =
[OH- ][B+ ] [B]
conjugate pairs: K w = K a K b
buffer composition
a buffer consists of a weak acid AND its conjugate base, or a weak base AND its conjugate acid. BOTH conjugates must be present. You cannot have a buffer with any strong acid and its conjugate or strong base and its conjugate. Buffers MUST come from weak acids and bases.
Henderson Hasselbalch
pH = pK a + log
[base] [acid]
Exam 2 · Practice Sets
Some nice old homework sets from Quest. We redid them and called them extra practice a few years ago. Here they are in the 2-column Quest pdf format. Try them all BEFORE you look at the key.
Acid Base 1 | KEY
Acid Base 2 |
KEY
Having that déjà vu moment? Some of our old (many actually) get copies over to Canvas.