Monday 7/19
8:30-10:00am
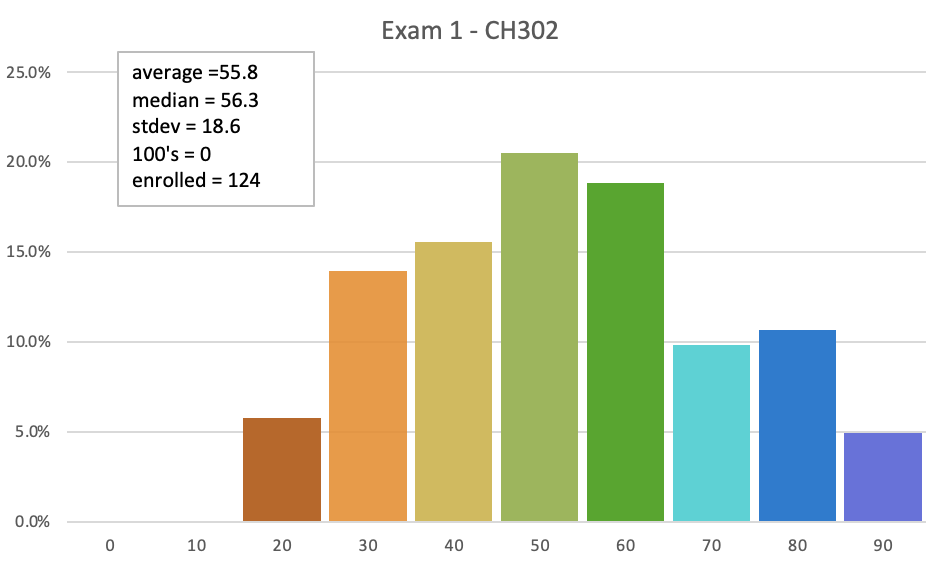
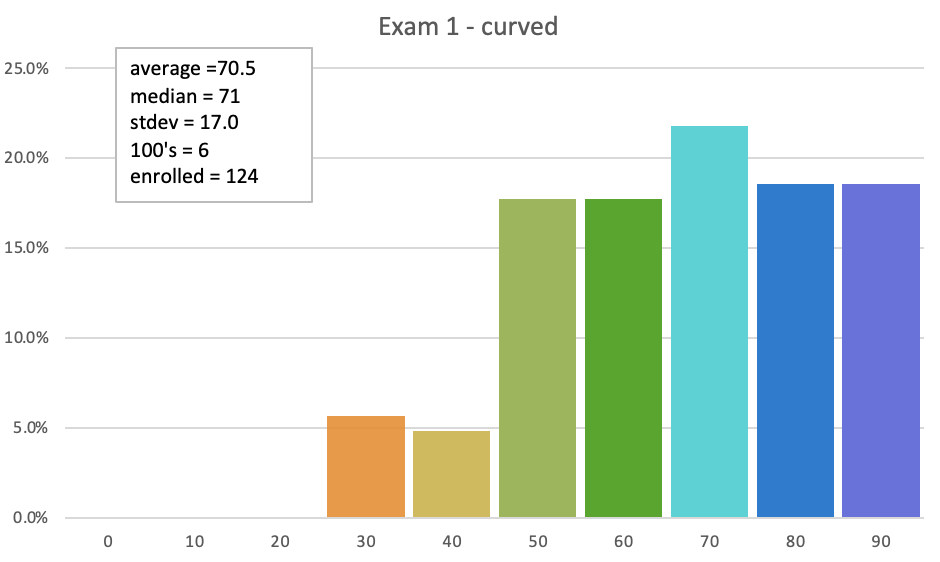
Students will be able to...
Students will be able too...
Common Chemistry Knowledge The following are common calculations that you have to know for any chemistry class. I'm a little embarassed including them - but better safe than sorry.
molecular weight =
mass of substance
moles of substance
molarity =
moles of solute
liters of solution
%conc =
mass of solute
mass solution
x 100%
molality =
moles of solute
kg of solvent
mole fraction of A =
moles of A
total moles
Main Equations/Formulas for Exam 1 Note that the student will need to know (memorize) all of the mathematical formulas for the exam. The Periodic Table Handout will only have constants, conversion factors, and data - no formulas.
\(q = m\cdot C_{\rm s}\cdot \Delta T\)
\(q = m \cdot \Delta H_{\rm transition}\)
\( \ln\left({P_2\over P_1}\right) = {\Delta H_{\rm vap}\over R}\left({1\over T_1}-{1\over T_2}\right) \)
\(\Delta H_{\rm solution} = \Delta H_{\rm lattice} + \Delta H_{\rm hydration}\)
\( C_{\rm gas} = k_{\rm H} \; P_{\rm gas} \)
\(P_{\rm solution} = \chi_{\rm solvent}\cdot P^\circ \)
\(\Delta T_{\rm f} = i\cdot k_{\rm f} \cdot m \)
\(\Delta T_{\rm b} = i\cdot k_{\rm b} \cdot m \)
\(\Pi = i\cdot MRT \)
Help Page on van't Hoff Factor
aA + bB ⇌ cC + dD
mass action =
activity, K
aCc aDd
aAa aBb
mass action =
conc, Kc
[C]c [D]d
[A]a [B]b
mass action =
press, Kp
PCc PDd
PAa PBb
Kp = Kc(RT )Δn
Note: remember to use 0.08206 L atm/mol K
for the value of R in this equation. And that...
Δn = (#mol gas products) – (#mol gas reactants)
Heating Curves
heating a substance: \(q = m\cdot C_{\rm s}\cdot \Delta T\)
Phase changes: \(q = m \cdot \Delta H_{\rm change}\)
Know how to calculate for various heating scenarios and phase changes. We WILL provide the heat capacities and enthalpies of change that are needed.
Vapor Pressure vs Temperature
Clausius-Clapeyron Equation: \( \ln\left({P_2\over P_1}\right) = {\Delta H_{\rm vap}\over R}\left({1\over T_1}-{1\over T_2}\right) \)
Your thinking here is that there are 5 variables in this equation. Somehow, we will give you 4 of them and you'll calculate the last one. Also remember... all normal boiling points have vapor pressures equal to 1 atm by definition.
Equilibrium Constant vs Temperature
van't Hoff Equation: \( \ln\left({K_2\over K_1}\right) = {\Delta H_{\rm rxn}\over R}\left({1\over T_1}-{1\over T_2}\right) \)
Looks almost identical to the CC equation above. However, ΔH in this one can be positive or negative.
Making Solutions (aka: Dissolving stuff)
Rule of Thumb: "Likes Dissolve Likes"
Which means that polar solvents tend to best dissolve polar substances and non-polar solvents tend to dissolve non-polar substances. If you have a mismatch (polar/non-polar), you are most likely going to have INsoluble substances - or put another way: the solubility will be very very low if there is a mismatch in polarities.
Dissolving Solids
When a solid does dissolve, the following must be true:
\(\Delta H_{\rm solution} = \Delta H_{\rm lattice} + \Delta H_{\rm hydration}\)
The lattice energy for a solid, \(\Delta H_{\rm lattice}\), is always +positive the way we use it (expanding the solid into separate molecules or ions). The hydration energy, \(\Delta H_{\rm hydration}\), is always –negative in the way we use it. Most salts when dissolving in water tend to have slightly bigger (magnitude) lattice energies than hydration energies which means that most salts have endothermic heats of solution (\(\Delta H > 0\) or + ).
Dissolving Gases
Because gases have no lattice energy, the heat of solution for all gases is exothermic (\(\Delta H < 0\) or – ). The solubility of a gas in a solvent is directly proportional to the partial pressure of the gas in contact with the solvent. That is Henry's Law. Quantitatively Henry's Law is:
Henry's Law: \( C_{\rm gas} = k_{\rm H} \; P_{\rm gas} \)
Vapor Pressure Lowering
Raoult's Law: \(P_{\rm A} = \chi_{\rm A}\cdot P_{\rm A}^\circ \) for volatile solvent A and non-volatile solute
Raoult's Law: \(P_{\rm total} = \chi_{\rm A}\cdot P_{\rm A}^\circ + \chi_{\rm B}\cdot P_{\rm B}^\circ \) for volatile solvent A and volatile solute B
Freezing Point Depression: \(\Delta T_{\rm f} = i\cdot k_{\rm f} \cdot m \)
Boiling Point Elevation: \(\Delta T_{\rm b} = i\cdot k_{\rm b} \cdot m \)
Osmotic Pressure: \(\Pi = i\cdot MRT \)
Remember: All concentration terms will need adjustment with the van't Hoff factor, \(i\), if the solute dissociates into ions (salts).
Want even MORE practice on Chemical Equilibrium problems? Well, here's a pdf of a set of extra practice problems (from spring 2019). Practice on these and see what you get / how you do. Then, have a look at the key and grade it. Note: these were from Quest, the CNS testing platform.