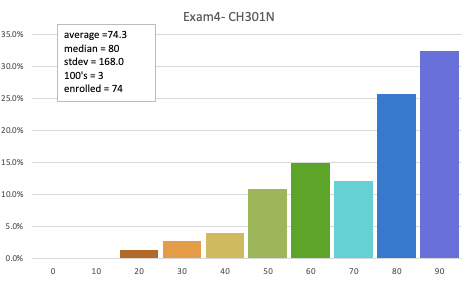
301N
 S22
S22
Exam 4
Wednesday 5/04
2-3:15pm
Learning Outcomes
Students will know...
- what the first law of thermodynamics is and how it is stated/used
- what the second law of thermodynamics is and how it is stated/used
- the difference in endothermic and exothermic reactions/processes
- the definitions of the system, surroundings, and the universe in thermodynamics
- the differences in an open, closed, and isolated system
- how to completely balance combustion reactions
- how to calculate the total energy available from a given amount of fossil fuel when heat of combustion data is given
- the mathematical relationships between heat, amount of substance, heat capacity, and temperature change for any given substance (the "mcat" formula)
- how to calculate heats of various processes using a calorimeter
- the difference in a bomb calorimeter and a coffee-cup calorimeter
- how to calculate the specific heat of a metal when given the needed calorimeter experimental data
- which fuels have the highest kJ/g energy output and the lowest (general trend)
- the general way in which fossil fuels are extracted from crude oil
- the purpose and reaction outcome of cracking and reforming on fossil fuels
- how to use a table of bond energies to calculate the heat (enthalpy) of a reaction
- how to calculate the heat in/out of a physical change in state (phase change)
- how to mathematically traverse a heating curve for a substance from solid state to gas state
Exam protocol All you need to know about HOW to take our in-class exams.