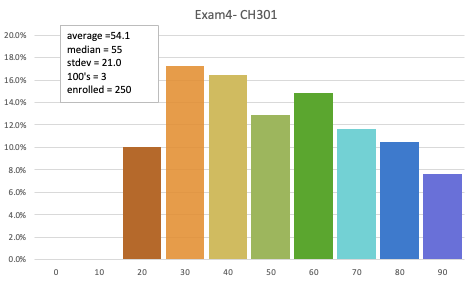
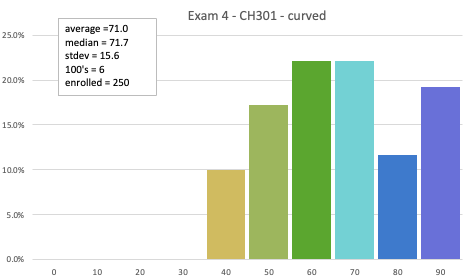
Exam 4
Wednesday 5/04
9:00am - 9:50am
Learning Outcomes
Students will be able to...
- Identify the system, surroundings, and universe in order to distinguish what is changing during a chemical and/or physical process.
- Define and recognize state versus process functions.
- Describe the concept of the energy units, including calories, kilocalories, and kilojoule.
- Distinguish between kinetic energy, potential energy, and electromagnetic energy.
- Define the first law of thermodynamics in the context of internal energy, heat, and work.
- Recall sign conventions associated with thermodynamic change.
- Define conduction and describe the microscopic view of thermal energy transfer due to molecular collisions.
- Define heat capacity, specific heat capacity, and molar heat capacity.
- Calculate the heat and work associated with chemical and/or physical change.
- Calculate \(P\Delta V\) (expansion) work for both physical and chemical changes.
- Define enthalpy, and calculate the enthalpy change for chemical and/or physical changes.
- Calculate change in enthalpy for physical change – both change in temperature and phase change.
- Fully interpret the heating curve of a substance.
- Draw and fully interpret energy reaction diagrams.
- Differentiate between the change in internal energy and enthalpy for a process, and describe how these quantities are measured (coffee cup vs. bomb calorimetry).
- Calculate the change in enthalpy (\(\Delta H\)) and internal energy (\(\Delta U\)) based on calorimetric data.
- Write formation reactions for elements and compounds.
- Calculate change in enthalpy based on tabulated data (e.g. Hess’s law, formation data, bond enthalpy data).
- Define entropy (\(S\)) and describe the second law of thermodynamics in the context of \(\Delta S\).
- Differentiate between the entropy of system, surroundings, and universe.
- Recognize how changes in system properties (\(T\), \(V\), phase, mixing, and composition) will affect the entropy of the system.
- Calculate change in entropy for the system and surroundings for a physical change.
- Describe entropy using a microscopic perspective of energy distribution (Boltzmann/microstates).
- Calculate change in entropy for the system and surroundings for a chemical change.
- Define the change in free energy.
- Calculate change in free energy (\(\Delta G\)) for a chemical change from change in enthalpy and change in entropy.
- Use the change in free energy to determine the spontaneity of a chemical and/or physical process at a given temperature.
- Calculate \(\Delta G\) for a chemical change from tabulated thermodynamic data.
- Link \(\Delta G\) to the second law of thermodynamics and chemical equilibrium.
Main Equations/Formulas for Exam 4
click on Smiley for an annotated list of equations! 
ΔU = Uf - Ui
ΔU = q + w
q = m Cs ΔT
q = n Cm ΔT
q = m ΔHtrans
q = n ΔHtrans
w = -PextΔV
w = -ΔngasRT
Δngas = (#mol gas prod) - (#mol gas react)
H = U + PV
ΔH = ΔU + PΔV
ΔU = ΔH - PΔV
ΔU = ΔH - ΔnRT
ΔH = qP
ΔU = qV
qcal = -qsys
qcal = CcalΔT
qcal = mwater · Cs,water · ΔT + Chardware · ΔT
ΔHrxn = ΔH1 + ΔH2 + ΔH3 + ...
ΔHrxn° = ΣnΔHf° (products) - ΣnΔHf° (reactants)
ΔHrxn° ≈ ΣnΔHbond°(breaking) - ΣnΔHbond°(making)
ΔSuniv = ΔSsys + ΔSsurr
S = k ln Ω
ΔS = qrev / T
ΔS = n Cm ln(Tf / Ti) molar version
ΔS = m Cs ln(Tf / Ti) mass version
ΔStrans = ΔHtrans / Ttrans
ΔSrxn° = ΣnS° (products) - ΣnS° (reactants)
G = H - TS
ΔG = ΔH - TΔS
ΔGrxn° = ΣnΔGf° (products) - ΣnΔGf° (reactants)
ΔH = TeqΔS

Flashback to Fall 2018
The creepy "eye" logo, the Welch renovation, having to teach in Burdine (oof)... oh yeah, the heart attack thing 💔 ... but I digress.
We also put up copies of all four exams before the final (both blank ones and the KEYS). The crazy thing is... I never took them off the old website! Don't tell anybody - it's a secret stash of the good stuff - Exam 4, the thermo exam is there in the stash. And, if you scroll down past the HONOR CODE blurb you will find another stash of thermodynamic practice problems - like 92 of them! Anyway, enjoy my Secret Stash from Fall 2018.
Exam 4 Worksheet...
Here is the worksheet (and KEY) that Claire and Kelly used for the Friday afternoon review.
Exam 4 Review Worksheet | KEY
Exam protocol All you need to know about HOW to take our in-class exams.