Exam 1
Thursday 2/13
2:00 - 3:15pm
UTC 2.102A
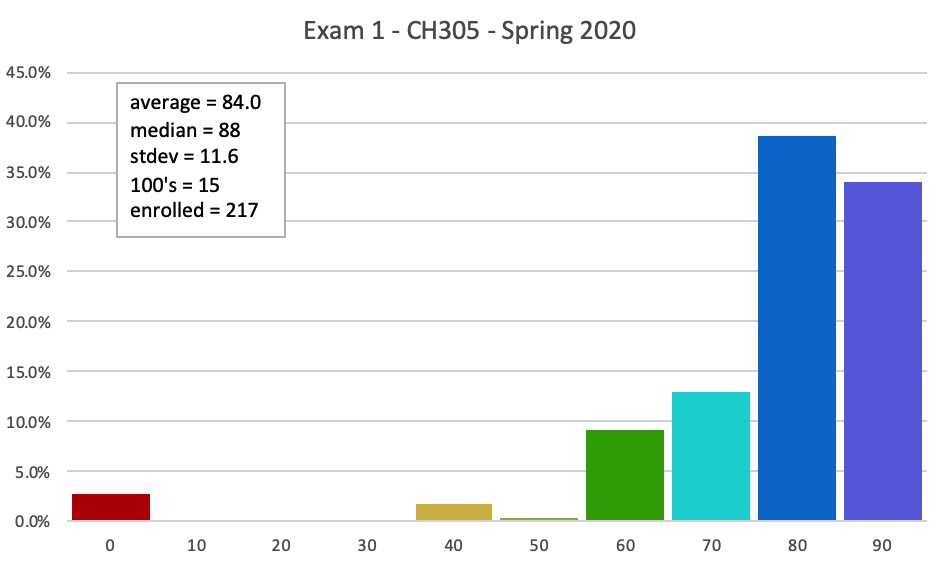
Here is the Answer KEY to all the versions of the exam.
Here is the MASTER KEY to Exam 1. Answers and explanations are shown, although the order will be different from yours.
Learning Outcomes
Students will know...
- how to count stuff
- how to mathematically convert from one type of unit to another utilizing a set of conversion factors
- the names, formulas, and physical state of the first 10 alkanes
- the MAIN Metric Prefixes for Chemistry Class as listed in section 10.2 of chembook - it's the last table there
- how to fully balance a chemical reaction and identify the coefficients
- how to balance an acid/base reaction
- the Arrhenius and Lowry-Bronsted definitions of acids and bases
- the names and formulas of the 7 strong acids and the 8 strong bases we covered
- how to identify the conjugate base of an acid and vice versa
- the definition and how to calculate the pH, pOH, and pK for solutions/substances
- what percent ionization is and how to apply it and calculate from it - predict pH
- how to convert a given concentration of a weak base or acid and the resulting pH into a percent ionization
- how to convert the concentration and percent ionization into a Ka or Kb
- what the definition of neutral water is
- how the pH scale works and how we describe the ranges - strongly acidic, distinctly acidic, slightly acidic, fairly neutral, and perfectly neutral - plus the analogous basic ranges as well
- how to identify conjugate acid/base pairs
- how to calculate the volume needed to reach the equivalence point of a given titration (neutralization)
- how to use titration data to determine the original concentration of a solution of acid or base
- what causes acid rain and how it can be prevented
- the chemistry of dissolving a salt into water
- the chemistry and results of dissolving CO2 into water/rain
- anything else we learned and did in class, on HW, that I forgot here
Here is a pdf helpsheet on the pH/pOH scale and the formulas for converting between H+ and OH– concentrations. This is pulled from the gchem site.
Formulas/Equations YOU should know
Kw = [H+][OH-]
pH = -log[H+]
[H+] = 10-pH
pOH = -log[OH-]
[OH-] = 10-pOH
%ionized =
[ionized] × 100%
[original]
weak acids / weak bases
acid reaction:
HA(aq) ⇌ H+(aq) + A-(aq)
Ka =
[H+][A-]
[HA]
base reaction:
B(aq) (+ H2O) ⇌ OH-(aq) + BH+(aq)
Kb =
[OH-][B+]
[B]
conjugate pairs: Kw = KaKb
