Exam 2
Tuesday 10/4
7:00pm - 8:30pm
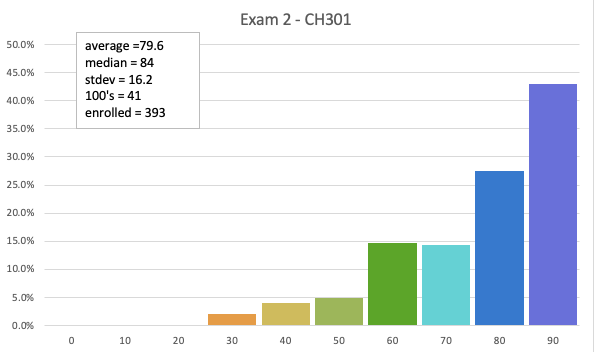
79.6 avg
🔑 Here are all the KEYS to Exam 2
Note: MOST (not all) Chapter 4 material REMOVED from Exam 2
Learning Outcomes for Atomic Theory (whole chapter - all sections)
Students will be able to...
- Perform quantitative calculations based on the relationship between wavelength, energy, and the speed of light.
- Identify and rank the different types of radiation which comprise the electromagnetic spectrum.
Explain why classical mechanics doesn't describe electromagnetic radiation.- Describe the photoelectric effect and relate the energy and/or intensity of the photons to the work function and kinetic energy of the ejected electrons.
- Explain the origin of atomic and emission spectra and relate these spectra to discrete energy levels.
- Apply the Rydberg formula to predict the energy of transitions between fixed energy levels in the hydrogen atom.
- Explain that quantum mechanics is a mathematical model, the solutions of which yield wave functions and energies.
- List the possible combinations of quantum numbers that are allowed.
- State the atomic orbital names based on quantum numbers.
- Know the basic shapes of the four types of atomic orbitals (s, p, d, and f).
- Know the number of nodal surfaces (planes, cones, and spheres) for the atomic orbitals.
- Explain that a wave function can be used to calculate a radial distribution function that describes the probability of an electron as a function of distance away from the nucleus.
- Distinguish between one-electron systems and multi-electron systems.
- Apply the Aufbau principle to determine the configuration for any atom or ion.
- Relate the electronic configuration of an element to its position on the periodic table.
- Recognize that there are exceptions to the Aufbau principle and predict where on the periodic table these are likely to occur. (I will not ask about this on the exam)
- Apply Hund's Rule and the Pauli Exclusion Principle to determine electron configuration using an orbital diagram (electrons in individual orbitals with spins).
- Fill an electron atomic orbital diagram and determine whether the element is paramagnetic or diamagnetic.
- Apply the shell model of multi-electron atoms to describe the concept of core vs. valence electrons.
- Describe the organization of the periodic table and the characteristics of elements in different regions of the table.
- Describe the concept of electronic shielding and effective nuclear charge (Zeff) and their relationship to trends in ionization energy, atomic radii, and ionic radii.
- Periodic Table Trends: ionization energy, atomic radii, ionic radii, and electron affinity. (PLEASE READ Chembook, Chapter 3 Section 10 on Periodic Table Trends - easy read, LOTs of good info)
Note: There are NO questions about black body radiation, UV catastrophe, or the Heisenberg Uncertainly Principle. Focus on what IS listed. As for wave particle duality... you do realize you are already doing this when you then about EM-rad as a wave (\(c = \lambda \nu\)) or as a photon (\(E = h\nu\)) - so that is all there is to know.
The chembook has a much better description of electron configurations than gchem (unless you watch all the videos there). At the bottom of the section is a sub-section on making cations and anions and how those species all tend to be isoelectronic with a noble gas. Read it! Learn it!
Actually... Read ALL of Chembook Chapter 3 - it is so much more approachable than what is on gchem. If you haven't been reading Chembook, you need to start on it.
Trends in the Periodic Table + Nomenclature of Ionic Compounds and Simple Covalents
Students will be able to...
- Identify metals and non-metals, and predict the types of compounds (ionic/covalent) that will form from different elements.
- Distinguish between molecules, ions, and atoms.
- Write out the correct electron configuration for any neutral atoms AND also for simple monatomic cations and anions.
- Recognize and identify isoelectronic species.
- Predict the anion or cation that a main-group element is likely to form.
- From the Fundamentals Chapter 1.7
- Nomenclature IONIC compounds: YES, you will be asked to name an ionic compound and also be given a name and you pick the correct formula. Yes, ALL the polyatomic ions are fair game here.
- Nomenclature COVALENT compounds: Name and write formulas for simple covalent compounds containing 2 different non-metals (like CO is carbon monoxide).
MOST (not all) Chapter 4 material (Bonding) has been Removed from Exam 2
You'll notice that HW07 was removed from the line up - that was Chapter 4 material.
Of course this means that all of Chapters 4 and 5 will be on Exam 3.
What you DO need from Chapter 4 is in 4.1 - section called ionic compounds and covalent compounds, 4.2 - most all of this section about ionic bonding and compounds, and 4.5 - electronegativity trends
Formulas YOU should know
electromagnetic radiation: \[c = \lambda \cdot \nu \]
single photon energy: \[E = h \cdot \nu \]
work function: \[E_{\rm k} = {1\over 2} m v^2 = h\nu - \Phi \]
Rydberg formula: \[\Delta E = {\cal R}\left({1\over n_i^2} -{1\over n_f^2}\right) \]
H Energy Levels: \[E_n = -{\cal R}\left({1\over n^2}\right) \]
The Rydberg constant
(\({\cal R}\)) is clearly labeled and given on the Exam Periodic Table Page for Exam 2 which is the front page of your exam copy. Actually, three values are given - use your favorite!
\({\cal R}= 2.18\times 10^{-18} \; \;{\rm J}\)
\({\cal R}= 3.29\times 10^{15} \; \;{\rm s^{-1}}\)
\({\cal R}= 1.097\times 10^{7} \; \;{\rm m^{-1}}\)
EM radiation
light : it's a wave! it's a particle!
Emission vs Absorption
emission: the electron drops down to a lower level and the equivalent amount of energy is released as a photon
absorption: the electron jumps up to higher level and the equivalent amount of energy is absorbed by the electron
Quantum Concepts
total nodes (nodal surfaces actually) in a given orbital \(= (n-1)\)
angular nodes (cones and planes) \(= \ell\)
spherical or radial nodes (spheres) \(= n - \ell - 1\)
Electron Configurations
use the periodic table to follow the Aufbau order
when making cations from transition metals - remove the \(s\) electrons first
Jimmy's Help Videos on Exam 2 topics
Exam protocol All you need to know about HOW to take our in-class exams.