
Course Archive
Course Archive (selected archived announcements)
Final Exam is OVER
Quest has finished grading
scores are there for you to see
Hopefully you remembered to take the final exam.
Calculate your GRADE in the course before I do...
Here is the Excel Spreadsheet for Calculating Your Overall Grade. Most UT students have Microsoft Excel. If you don't, then go to Sheets in docs.google.com and open it in there. Google docs will read Excel files just fine. Of course you need a google account and doing it in Chrome helps as well (I couldn't get Safari to allow the upload). - Dr. McCord
You'll need to enter 8 total scores into the spreadsheet:
Five from Quest: Exams 1-4 and Final*
Three from Canvas: HW Avg, TopHat Avg, and Quiz Avg
* remember to add 2 more points to your Final if your eCIS Completion score on Canvas is 2
Final Exam is OVER
10am class final is OVER
11am class final is OVER
There is a BIG point penalty for missing the final exam! Don't miss the exam and make sure of your time and room.
Exam has 50 questions and all questions are 2 points each.
The front page (cover page) of the exam is a formula page.
Here is the Front Page to the Final Exam
Version Numbers on Quest, or...
On Quest, the Grade on the assignment "Final version" is your version number. Room assignments are also listed on Quest as an announcement.

11am Class ONLY! 9am Wed
all versions 001-408 in WEL 1.308
Here is the Excel Spreadsheet for Calculating Your Overall Grade. Most UT students have Microsoft Excel. If you don't, then go to Sheets in docs.google.com and open it in there. Google docs will read Excel files just fine. Of course you need a google account and doing it in Chrome helps as well (I couldn't get Safari to allow the upload). - Dr. McCord
All non-exam Scores on Canvas Now
Thank you to the +90 students who put their UTEID on Sapling and Canvas. All scores have now been updated on Canvas and are FINAL. Students who did not do this got zeros.
There are FOUR important scores to get off of Canvas. They are: TopHat Avg, Quiz Avg, HW Avg, and eCIS Completion. Use those in the grade spreadsheet along with your exam scores from Quest to enter into the Calculate your Grade spreadsheet given just below this announcement. You will just ADD the eCIS completion points to your final exam score from Quest. Those points will NOT show in Quest.
Exam 4 Scores on Quest
there will be no curve on this exam
Email Kristin about your zero you got because you didn't bubble in your version number or uteid correctly.
Double Check your UTEID in Sapling
If your current score on Canvas is a ZERO, then you need to do this. Do it and wait. I'll download again and recalculate on Sunday afternoon.
Click on "Profile", and then next screen click the "Edit profile" tab. Scroll way down to "Optional" and enter your uteid in the Student ID field.
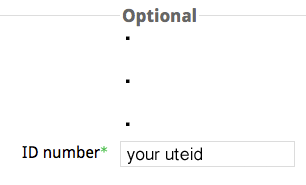
Double Check your UTEID in Top Hat
If your current score on Canvas is a ZERO, then you need to do this. Do it and wait. I'll download again and recalculate on Sunday afternoon.
Via Web: Go to our class. Top menu bar under your name click "My Account". Left menu click "Grading Setup". Enter your uteid into the field presented.
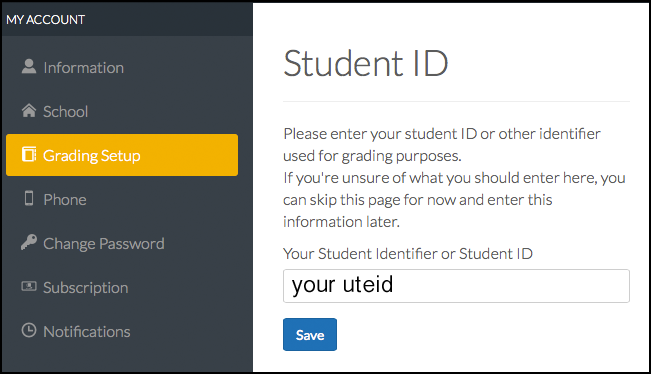

DEADLINE PAST! - NO MORE CREDIT ON THIS
Incentives for doing the eCIS
Getting an extra +2 points on your final exam next week simply by completing the eCIS and uploading your screen shot of the "Completion" page. Click the big-ass graphic to the left and you'll be taken to Canvas where you can follow the directions about the upload.
Deadline on this is Friday, 5/8 at 9pm 11:59pm
Just to be Perfectly Clear...
In today's class I wrote the cell notation for both the PbO2/PbSO4 cell and the Pb/PbSO4 cell which make up the Pb-acid car battery. I wrote "anode" on one and implied that was for the battery in use - well if you put the PbO2 on the left, then you are writing the electrolytic cell (Pb-acid on REcharge). Anyway here are BOTH versions of that cell and the 1/2 reactions.
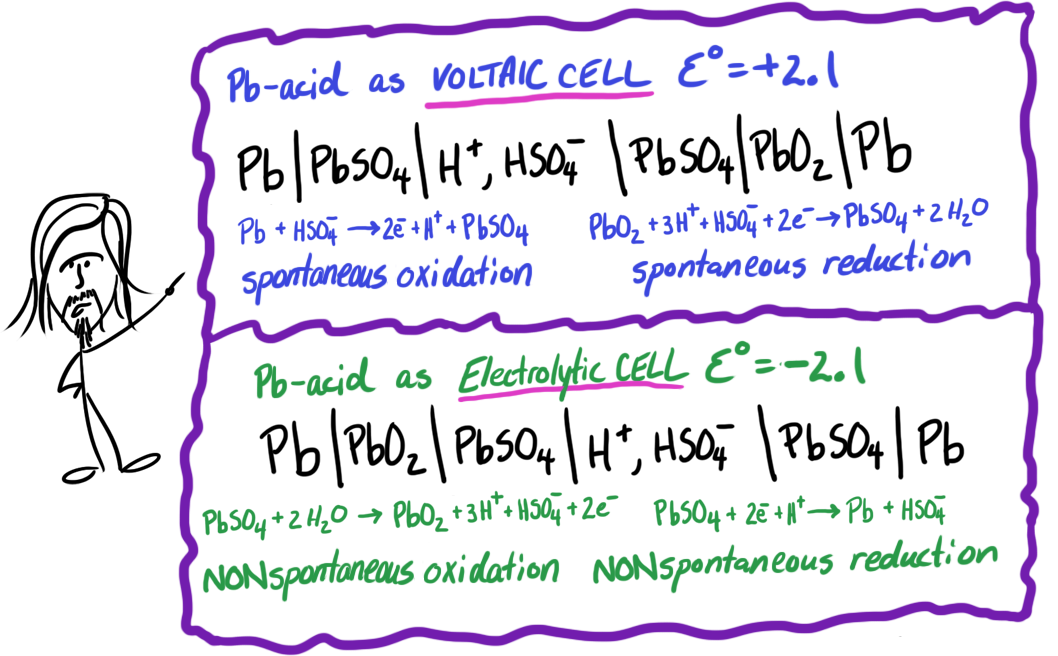
Note that one is just the flipped version of the other. Do know which way is which though.
Exam 4 Thur, 5/7, 7-9 PM
Exam has 30 questions (plus the "extra credit" question which now has only one choice to bubble). Most questions are 4 points, althought there are a few 2 point and 3 points questions as well.
The front page (cover page) has a table of standard potentials for you to use.
Version Numbers are HERE --- deactivated ---
Your VERSION NUMBER determines the ROOM
You can also check Quest for your "grade" on the assignment "Exam4 version". That is your version number for Exam 4. Now look below and find which of the 2 rooms you'll need to go to.
versions 001-230 in WEL 2.224
versions 231-420 in WEL 1.316
Early Takers: Once again, those students with documented conflicts will take the exam from 4-6pm in WEL 3.502.
Practice Exam for Unit 8 is now on Canvas
Unit 8 Practice Exam - Multiple Choice is due by 11:59pm on Wed, 5/6
Unit 8 Practice Exam - Free Response is due by 11:59pm on Wed, 5/6
Q32 - Redox Reaction is due by 4:20pm on Mon, 4/20
Q33 - Electrochemical Cells Intro is due by 10am on Wed, 4/22
Q34 - Standard Potentials is due by 10am on Thu, 4/23
Q35 - Electrochemical Stoichiometry is due by 10am on Mon, 4/27
Q36 - Free Energy and Work is due by 10am on Tue, 4/28
NEW SECTION! Ksp from E° so new it didn't make it into Q36! Now on Q37 (last question) - Please read and know how this works - definitely an exam possibility!
Q37 - Non-Standard Cell Potentials is due by 10am on Thu, 4/30
Q38 - Batteries is due by 10am on Mon, 5/4
HW12: Oxidation/Reduction is due by 4:20pm on Mon, 4/20
HW13: Electrochemistry 1 is due by 10am on Tue, 4/28
HW14: Electrochemistry 2 is due by 5pm on Mon, 5/4
Behold, the Triforce of Chemistry!
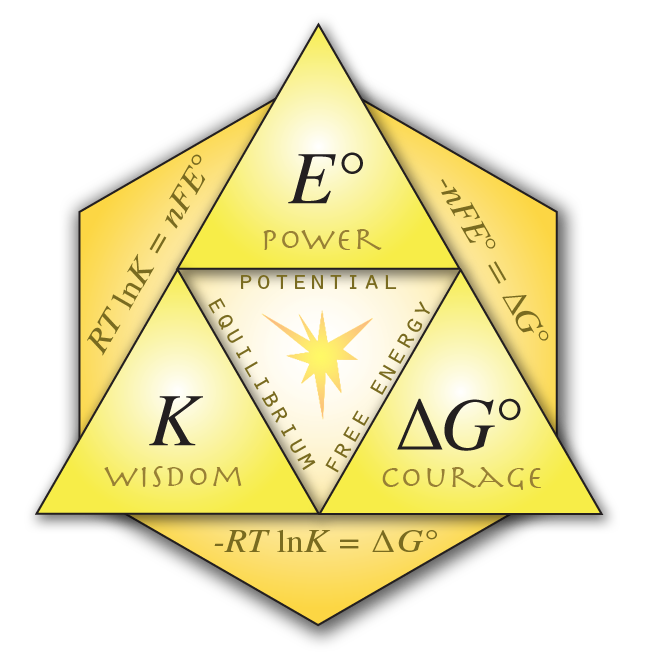 click to enlarge
click to enlarge
Nernst Equation:
\(E = E^\circ - \left({RT\over nF}\right)\ln Q\)
It works for 1/2 reactions! It works for whole reactions! It's amazing!
\(E = E^\circ - \left({0.0257\over n}\right)\ln Q\)
\(E = E^\circ - \left({0.05916\over n}\right)\log Q\)
More Equation Goodness
\(RT \ln K = nFE^\circ = -\Delta G^\circ\)
\(RT/F = 0.0257\) good for use with ln
\(RT/F \cdot \ln(10) = 0.05916\) good for use with log
\(K = e^{(nFE^\circ/RT)} = e^{(nE^\circ/0.0257)} = 10^{(nE^\circ/0.05916)}\)
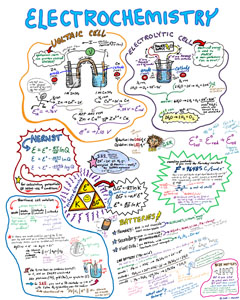
The
Super-Doodled
Electrochemistry
Help
Sheet
Exam 3 Scores are Available on Quest!
Contact Kristin if you have a zero on Exam 3 and you know you took the exam - she is making Quest corrections.
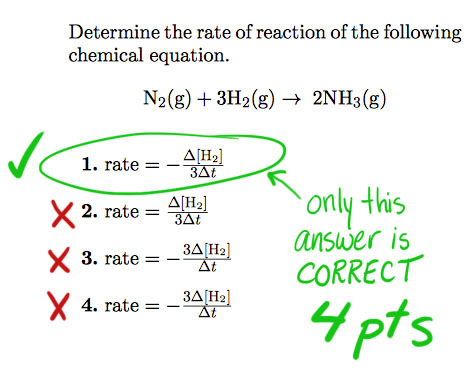
THE CORRECTION on QUEST - do know that the problem shown below was/is flagged with the wrong "correct" answer in Quest. Each student who picked the correct answer (shown below) got 4 points in their tally. Please LOOK at this before you email with your concern.
Exam 3 Thur, 4/16, 7-9 PM
Start thinking, preparing, and focusing on Exam 3!
Exam has 33 questions (plus the "extra credit" question which now has only one choice to bubble). Many of the questions are 2 points and should be very fast to answer.
Version Numbers on Quest, or...
--- deactivated ---
On Quest, the Grade on the assignment "Exam3 version" is your version number. Room assignments are also listed on Quest as an announcement.
RE-OPENED!!
Unit 7 Practice Exam - Multiple Choice is due by 11:59pm on Wed, 4/15
Unit 7 Practice Exam - Free Response is due by 11:59pm on Wed, 4/15
Your VERSION NUMBER determines the ROOM
Check Quest for your "grade" on the assignment "Exam3 version". That is your version number for Exam 3. Now look below and find which of the 2 rooms you'll need to go to.
versions 001-240 in UTC 2.112A
versions 241-430 in UTC 2.102A
Early Takers: Once again, those students with documented conflicts will take the exam from 4-6pm in WEL 3.502.
"I Love Lucy" teaches Kinetics
A very important concept in chemical kinetics is the "rate determining step". It is easy to spot in many situations - like here with Lucy and Ethel (although, we chemist prefer "Ethyl"). The overall rate law is governed by the slowest step in any multistep reaction scheme.
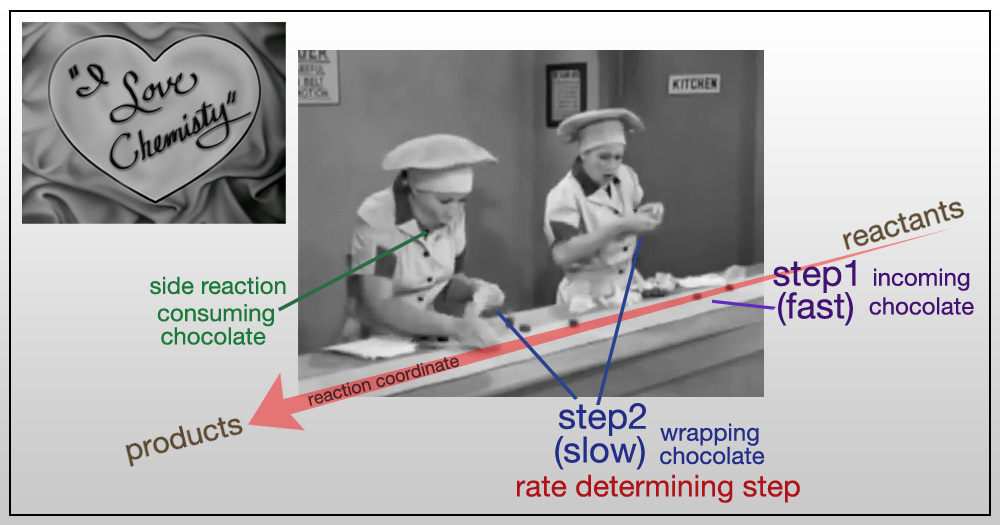
Here is Dr. McCord's Kinetics Review Sheet.
 Happy Easter!
Happy Easter!
Q28 bumped all the way to Sunday night at 10pm
Friendly Reminder: Drop Deadline is Monday, 4/6

Q24 - Nuclear Chemistry is due by 10am on Wed, 3/25
Q25 - Radioactivity is due by 10am on Fri, 3/27
Q26 - Nuclear Applications is due by 10am on Mon, 3/30
Q27 - Reaction Rates - Rate Laws is due by 10am on Thu, 4/2
Q28 - Integrated Rate Laws is due by 10pm on Sun, 4/5
Q29 - Reaction Mechanisms is due by 10am on Wed, 4/8
Q30 - Arrhenius Theory is due by 10am on Fri, 4/10
Q31 - Catalysts is due by 10am on Mon, 4/13
HW09: Nuclear Chemistry is due by 10am on Wed, 4/1
HW10: Kinetics 1 is due by 10am on Tue, 4/7
HW11: Kinetics 2 is due by 10am on Mon, 4/13
Spring Break is OVER

time to get your game face on!
26 days to learn nuclear chemistry and chemical kinetics. Exam 3 will be here before you know it.
Exam 2 - Grades on Quest
You can login to Quest and see your Exam 2 grades.
There will not be a curve on Exam 2.
Exam 2 Thursday, 3/12
Start thinking, preparing, and focusing on Exam 2 on Thursday!
Exam has 28 questions. 10 questions x 4 points each = 40 points for non-aqueous equilibria. 18 questions (various points) = 60 points for aqueous acid/base theory and equilibria.
Version Numbers on Quest, or...
--- deactivated ---
You can also find your version number on Quest. The Grade on the assignment "Exam2 version" is your version number. Room assignments are also listed on Quest as an announcement.
Unit 6 Practice Exam - Multiple Choice is due by 11pm on Wed, 3/11
Unit 5 Practice Exam - Free Response is due by 11pm on Wed, 3/11
Your VERSION NUMBER determines the ROOM
Check Quest for your "grade" on the assignment "Exam2 version". That is your version number for Exam 2. Now look below and find which of the 2 rooms you'll need to go to.
versions 001-222 in WEL 2.224
versions 223-444 in BUR 106
Protonated vs Deprotonated states

"I'm a protonated state because I HAVE the proton." You might know me best as a plain acid, HA. But remember, I can also be a protonated base, BH+. It doesn't matter which version I am, I still behave as an acid and I donate this proton to a base. My acidic strength is measured by my value for Ka.

"I'm a deprotonated state because I don't have the proton." You probably know me best as a plain base, B. I also exist as a deprotonated acid, A-. I will always behave as a base because I am ready to accept a proton from an acid. My base strength is measured by my value for Kb.
If the only difference between me and my buddy there is that one single proton, then we are a conjugate acid/base pair and have a special relationship which is that: Ka· Kb = Kw
Q13 - Equilibrium Constants is due by 1pm on Tue, 2/17
Q14 - Free Energy and Equilibrium is due by 1pm on Tue, 2/17
Q15 - Le Chatelier's Principle is due by 10am on Thu, 2/19
Q16 - Intro to Acids and Bases is due by 10am on Fri, 2/20
Q17 - Weak Acids and Bases is due by 10am on Mon, 2/23
Q18 - Ka, Kb, Kw is due by 10am on Wed, 2/25
Q19 - pH and pOH is due by 10am on Wed, 2/25
Q20 - Neutralization and Salts is due by 10am on Mon, 3/2
Q21 - Buffers is due by 10am on Mon, 3/2
Q22 - Titrations is due by 10am on Wed, 3/4
Q23 - Protonated States is due by 10am on Fri, 3/6
Unit 6 Practice Exam - Multiple Choice is due by 11pm on Wed, 3/11
Unit 5 Practice Exam - Free Response is due by 11pm on Wed, 3/11
HW05: Chemical Equilibrium Intro is due by 10am on Thu, 2/19
HW06a: Chemical Equilibria II is due by 10am on Mon, 2/23
HW06b: Acid/Base Equilibria I is due by 10am on Fri, 2/27
HW07: Acid/Base Equilibria II is due by 10am on Wed, 3/4
HW08: Buffers & Titrations is due by 5pm on Tue, 3/10
HW07 now due on Wednesday at 10am
If you already busted your ass to complete it, then good for you, thumbs up, way to go, you are awesome, and more students should be like you. Maybe you can start on HW08 tomorrow. See you in class on Wednesday and we WILL be talking 'bout buffers and titrations. - Dr. McCord
Four Problems Solved the same way...
SO MANY Problems in acid/base theory are answered via the following generic set up.
\[K = {x^2\over C - x}\]
K will either be Ka or Kb and you will typically solve for x and it will be either [H+] (acid problems) or [OH-] (base problems). You then take the log of x and you'll have either pH or pOH.
IF K is small enough (say < 10-4), then the following approximation is valid:
\[K \approx {x^2\over C}\]
and easily solved without using the quadratic formula...
\[x \approx \sqrt{K\cdot C}\]
Example wordings of questions that ultimately solve like shown above
- What is the pH of a 0.??? M solution of ?????
- What is the percent ionization of ???? in a solution of 0.??? M ?????
- What percentage is protonated in...
- What percentage is deprotonated in...
- Given that a solution of 0.??? M of ???? has a pH of ??.??, what is the value of K?
Oh, Megan...
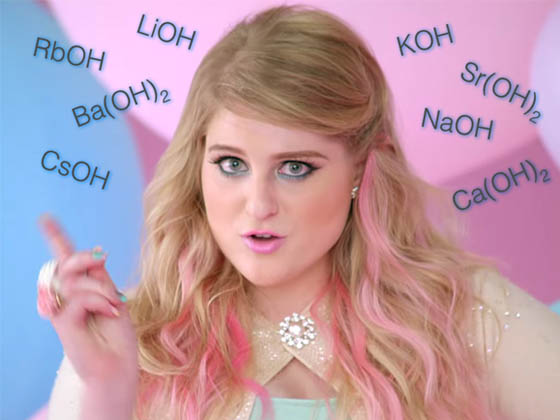 You really should know it's all about that ACID as well.
You really should know it's all about that ACID as well.
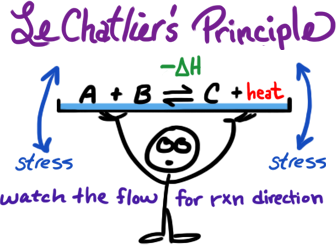 * example shown is for exothermic only
* example shown is for exothermic only
K is where you are going.
Q is where you are at.
Know the way you are heading
from the state that your system is at.
if Q < K shift right →
if Q > K ← shift left
if Q = K no change ⇌

Fun Facts with Acids and Bases! best that you memorize all of these!
- Acids have the protons and "throw" them to bases which "catch" them. Throw/Catch... donate/accept.
- Acidic solutions turn litmus red or pink / Basic solutions turn litmus blue.
- The pH scale is based on the H+ concentration : pH = -log[H+] -or- pH = -log[H3O+]
- A neutral solution is one where [H+] = [OH-]
- At 25°C, neutral pH is equal to 7.00
- Strong acids and bases are strong electrolytes, meaning 100% dissociated in solution.
- The strongest possible acid in aqueous solution is the solvated proton, aka: hydronium ion, H3O+
- The strong possible base in aqueous solution is the hydroxide ion, OH-
- The hydronium ion is functionally equivalent to the proton or hydrogen ion in aqueous solutions.
- Weak acids and bases are weak electrolytes, typically less than 5% dissociated in solution
- All acids have one and only one specific conjugate base and all bases have one and only one specific conjugate acid. Those couples are known as conjugate pairs.
- Weak acids have Ka's and weak bases have Kb's
- Buffers are made from weak acids and their base conjugates or weak bases and their acid conjugates.
- Polyprotic acids have as many Ka's as they have acidic protons. They are numbered sequentially, Ka1, Ka2, Ka3, etc...
Memorize this lovely poem I just wrote. Yes, I rhymed "at" with "at" but that is ok - I've listened to a lot of rap and they do that.
K is where you are going.
Q is where you are at.
Know the way you are heading
from the state that your system is at.
Chemistry Valentines...
I played this keynote presentation in class today. Now it's a video you can watch. Happy Valentines Day. - Dr. McCord
Exam 1 Scores are on Quest
Breaking News!: Exam1 being bumped up by 4 points via the extra credit question. You DID answer that you wanted the points, right? If you bubbled "no", then you got exactly what you asked for.
You can see your score and the key pdf to your exam. Remember in Quest the tally column over on the right side has YOUR responses listed.
Class average was a 72
Practice Exams Available on Canvas
Go to Canvas and look at the last 2 items in the module for Unit 5 and you will find 2 practice exams for Unit 5. That IS the exam used last year. However, it is a timed exam. Please use it to truly test yourself.
One of the exams is the free response section from the Vanden Bout class last year. We (McCord) will not have a free response section, but you should still go through it because the material there is relevant.
DIRECT LINKS to the 2 exams are also available from this page. Scroll down to the "Canvas Section" and you'll see the links at the end.
To help out students taking the exam earlier, the "due time" is now 2pm - however, the exams will stay open all the way to 7:20pm. In theory, this means that all students can see the solutions starting at 2pm... but the assignment is still useable/takable till 7:20pm which is after you should already be knee-deep in the actual exam.
McCord is sad like Keanu...

A true apology from me and Keanu about those questions from HW04 that made you sad. There are lots of other words that come to mind I'm sure. To remedy some of the heartbreak on this, I have made those questions have a weighting of ZERO on HW04. You can still look at them - but they will count nothing. I'll even do one better, none of those topics are on Exam1, zilch. So please, like the song says, "Let it Go". - Dr. McCord
Other quotes about HW04...
Direct tweet to PbI2 from Tom Petty, "don't come around here no more!".
"Those questions were not artistic, you should remove them and give them to Beyonce." - Kanye West
"Those questions left me deflated." - Tom Brady
"you've been chopped!" - Ted Allen
Exam 1 Thursday, 2/12
Start thinking, preparing, and focusing on Exam 1 on Thursday!
Version Numbers on Quest, or...
--- deactivated ---
You can also find your version number on Quest. The Grade on the assignment "Exam1 version" is your version number. Room assignments are also listed on Quest as an announcement.
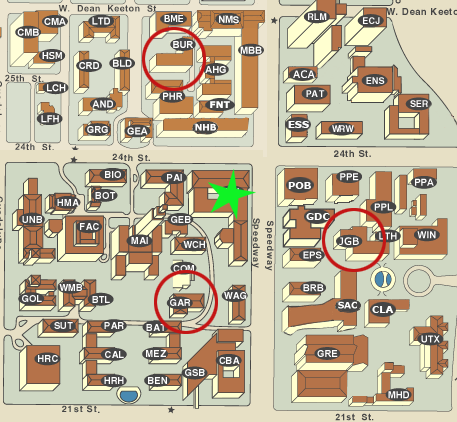
Your VERSION NUMBER determines the ROOM
Check Quest for your "grade" on the assignment "Exam1 version". That is your version number for Exam 1. Now look below and find which of the 3 rooms you'll need to go to.
versions 001-120 in JGB 2.324
versions 121-210 in GAR 0.102
versions 211-470 in BUR 106

NEWS FLASH!
Pure solids and pure liquids have no meaningful concentration term. Their activities are always exactly 1. This means that they are NEVER part of a mass action expression or equilibrium expression. LEAVE THEM OUT!!
Concentrations only make sense for dissolved species (think "aqueous" phase) or for gases (think pressure, which is really a concentration term for gases).
ALL Quizzes and Homeworks now Available
The remainder of the quizzes for Unit 5 are ALL accessible on Canvas. The same is true for the homeworks on Sapling. All you go getters can go and get it now.
Register for Sapling Learning
Go to the Canvas site and go to Modules. Click on the "Sapling Registration" assignment. Read. Then click and follow directions.
Your UTEID is your Student ID for everything!!
Any ISSUES you have with getting properly registered with Sapling Learning and "into" our homework, please contact Sapling, not Dr. McCord. Use the link below to see their FAQ page - an email link is there for support.
Register for Top Hat
IF you were on roster for class on or before 1/19/2015: You should've gotten an email from Top Hat with an invite. Click on the link there and follow the directions.
IF you ADDED this course AFTER the invite: Or, anybody else that wants to join our Top Hat class. You do NOT have to have the invite to join. You only need to "find" the course and add it in Top Hat. Searching for "McCord" and "Spring 2015" will most likely work at finding it. Here is the sure fire way...
To add the 10am course, use the TopHat course code: 695444.
To add the 11am course, use the TopHat course code: 613030.
Q00 - Welcome to CH302 is due by 10am on Wed, 1/28
Q01 - Unit 5 Intro is due by 10am on Wed, 1/28
Q02 - Phase Changes and Thermodynamics is due by 10am on Fri, 1/30
Q03 - Heating Curves is due by 10am on Fri, 1/30
Q04 - Phase Diagrams is due by 10am on Fri, 1/30
Q05 - Vapor Pressure is due by 10am on Fri, 1/30
Q06 - Solutions is due by 10am on Mon 2/2
Q07 - Solution Concentrations is due by 10am on Mon 2/2
Q08 - Henry's Law is due by 10am on Wed 2/4
Q09 - Colligative Properties is due by 10am on Wed 2/4
Q10 - Ionic Equations is due by 10am on Fri 2/6
Q11 - Ion Product is due by 10am on Tue 2/10
Q12 - Common Ion Effect and Precipitation is due by 10am on Tue 2/10
Practice EXAM 1 - Multiple Choice is due by 8pm on Wed 2/11
Practice EXAM 1 - Free Response is due by 8pm on Wed 2/11
HW00: Fundamentals is due by 10am on Wed, 1/28
HW01: CH301 Review is due by 10am on Wed, 1/28
HW02: Physical Equilibria is due by 10am on Mon, 2/2
HW03: Solutions / Colligative Properties is due by 10am on Fri 2/6
HW04: Solubility Equilibria is due by 4pm on Mon 2/9
McCord's Spring 2015 - CH302 Website
Website is now working. There will continue to be some more tweaks between now and Friday. Right now you need to get signed up and registered for Sapling Learning (homework) and for Top Hat (in class questions and quizzes).
The first day of class is Wednesday, January 21st for this course.


