stash
Question: Where do all the old announcements and links go after they disappear from the front page?
Here, on the stash page.
All Grades are IN Now
Happy Holidays
Good Luck in your future studies
I bid you all adieu...
Yes, I got a LOT of emails about grades. Various pleas and the like. Like I said in class, I read all of them and did not respond because really what would I say? All grades were assigned blindly to the students names - just a bunch of numbers which is what we go by here. Many of you will be pleasantly surprised and got your wish while others didn't quite make the cut they wanted. All students were put through the same scrutiny and general fairness as all other students. So whether you sent an email or not really didn't come into play as far as grading.
Now that the grades are done, I might re-read a few emails and maybe even respond to a few. I did appreciate some of the humor and creativity in many of the emails. Thanks for a good and memorable semester - one I'm lucky to have finished in hindsight. For those that say bye to chemistry for good, best of luck in those other endeavors. For those that will continue on with organic chemistry, good luck to you - it is a whole different game than general chemistry. And to all...
Hook 'em,
- Dr. McCord
ALL scores are in Canvas now
I have imported exam4 scores into canvas. This means that all needed scores are in canvas now except your final exam score (and the eCIS score). That IS my gradebook and you can see your scores. ANY issues with those scores must be pointed out before final exams start. This is your responsibility to CHECK and make sure there are no error/omissions.
- Dr. McCord
Blank Exams and KEYS
Below are copies of our four exams from this semester. These are version numbers that were not used and therefore "new" to you as far as the ordering goes. Use these as one of your many ways to study up for the final exam. The keys are also provided.
Final Exam HANDOUT
Remember that you will get a periodic table handout with your final exam test copy. It will have the usual information on the front and a whole page of formulas on the back. This is shown on the gchem site. Or, have a look below for the quick links:
Periodic Table Handout (FRONT)
Periodic Table Handout (BACK - formulas)
(these are all the formulas we are giving you)
Your Final REEF Score is up
I have updated your Reef score in Canvas for the last time. There were 35 total sessions and I dropped your 6 lowest sessions.
Practice Sets from 2013
Way back in the day all our assignments were in Quest. Here are four assignments with their respective keys from back then. Use them to hone your kinetics and electrochemistry skills as you prepare for the exam on Wednesday.
Kinetics 1 | KEY
Kinetics 2 | KEY
UT Honor Code
“As a student of The University of Texas at Austin, I shall abide by the core values of the University and uphold academic integrity.”
Solutions to Exam 3 - Bonus
Many of you have asked for the solutions to "Exam 3 - Bonus" that was given on Canvas. Here you go:
Hmmm... #22 on HW07?
I'll show you how to work it. I'll also tell you that I will not put anything like it on the exam or final. The trick is writing the Arrhenius equation twice, one for the catalyzed reaction and one for the non-catalyzed reaction and then ratio-ing them. Like this...
\({k_c\over k} = {A e^{-E_{\rm a,c}\over RT}\over{A e^{-E_{\rm a}\over RT}}}\)
the \(A\)'s cancel out, then take natural log of both sides...'
\(\ln\left({k_c\over k}\right) = {E_{\rm a}\over RT} - {E_{\rm a,c}\over RT} \)
\(\ln\left({k_c\over k}\right) = {1\over RT}(E_{\rm a} - E_{\rm a,c}) \)
\( RT\ln\left({k_c\over k}\right) = E_{\rm a} - E_{\rm a,c} \)
\(E_{\rm a,c} = E_{\rm a} - RT\ln\left({k_c\over k}\right) \)
Where the subscript "\(c\)" is for the catalyzed reaction. For number 22 on HW07, you are told the catalyzed reaction is 655 times faster which means \(k_c/k\) is 655. The rest is fairly straight forward.
The main issue I have with this problem is the statement in the question "...all other factors being equal". THe fact is that once you catalyze a reaction, all other factors are in fact NOT equal, which means specifically the value for \(A\) would be considerably different and would not cancel out. This is still a nice exercise in math skills and logic though - so we ARE leaving it on the assignment.
Exam 3 - Scores Available
Sat 11/9, 9:00am: I have decided to curve the exam. Details are on Quest and also on the new canvas assignment "Exam 3 - Curved". That will be your score for Exam 3 if you do nothing else. However, IF you are successful on the canvas bonus quiz which will be discussed in class on Monday - then you can raise that score another 8 points.
Sat 11/9, 8:50am: Quest has made it through the grading and we have corrected all the bubblesheet errors that we could. The results are terrible but they are what they are. You can now view both your raw score and the solutions on Quest now.
Fri 11/8, 10:22pm: Still no word on the state of grading for the exam. I guess we will wait overnight and hope for better news in the morning.
Fri 11/8, 1:30pm: Quest is having difficulty grading the exam today. The Quest admin team has identified the problem and is on it. This does mean that it will be a somewhat longer wait on the scores. They are hoping to get something working by this evening. Stay tuned. I'll update this message if anything new has happened.
Dr. McCord
Acid/Base Trainer Page
Only work on the first two options listed on this page for Exam 2 - the rest of the options are for buffer problems and titrations (Exam 3). Specifically, the links titled "pH of a Weak Acid or a Weak Base" and "pH of the SALT of a Weak Acid".
Here is the URL: http://mccord.cm.utexas.edu/courses/acidbase/trainer/
Correction for 10/03 Notes
This equation: \(K_{\rm p}=K_{\rm c}(RT)^{\Delta n}\)
The value to use for \(R\) is 0.08206 L atm/mol K. The notes (doodles) for 10/03 have also been corrected.
Looking for HW PDFs ?
I have created a whole new page to list all the HW pdfs for the semester. It's currently on the new bottom row of icons above with a briefcase icon .


Now on to Unit 2 / Module 2 - Chemical Equilibria
LE08 - LE11 and HW03 are all available on Canvas now.
Here is a link to a YouTube Video on the [Co(H2O)6]2+ to CoCl42- reaction that I demo'd today, 9/21 Note: according to many of the comments, they should've left off the backing music track. Science is good if you can overcome the musics
Newly Available! A Help Page on the van't Hoff factor
Exam 1 is Wednesday, 9/19
Click on the Exam 1 link above to get all the details.
Geoffrey's Exam 1 Review PowerPoint is available in Reviews
In case you missed it or want to share, here is a link to my guest editorial for the Daily Texan on Monday. Maybe it will make you smile?
Textbook Questions? Here is a whole page on that. Give it a look if you are concerned: Buying a Textbook for CH301 and CH302 - some things you should consider and know.
Yes, I do use iClicker Reef - you will need an iClicker Reef subscription for the term which amounts to $14.99 for a 6-month subscription.
AND, just to be perfectly clear...
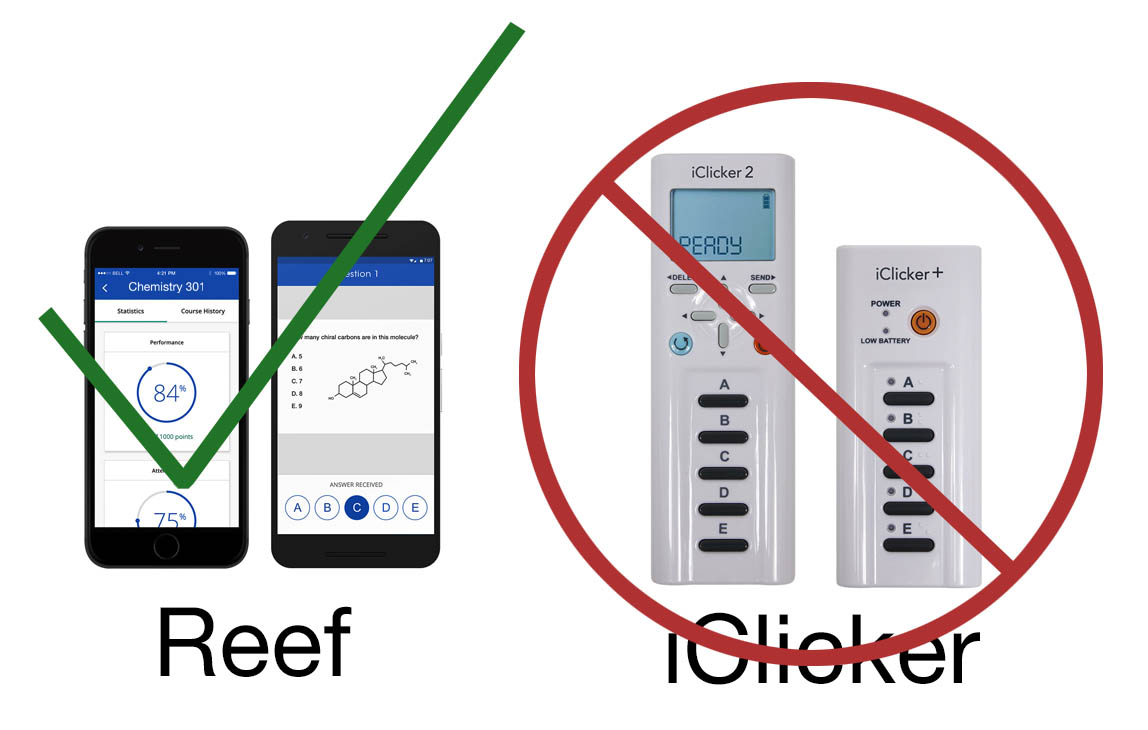 And to spell it out for you... I do not at all use iClicker remotes in class nor will they work if you bring them. Use them for biology class.
And to spell it out for you... I do not at all use iClicker remotes in class nor will they work if you bring them. Use them for biology class.
No, there is no course-pack for this course.
Greetings...
Yes, this is my official website for CH302. Click around if you want. Come to class on Wednesday and find out more. Time to get this party started.
- Dr. McCord