Partial Review Sheet for Exam 3 - McCord CH301
version 1 - posted on 11/04/12 at 9:05 AM
A Fair Warning about this Review Sheet
This "review sheet" is provided as is. Most all of it was written last year when we used the Zumdahl textbook. I've tried to remove all references to that book here. The information contained here is all valid although it's a bit too "McCord-centric" to stick it in the eBook right now. DO check the Learning Outcomes for Unit 3 for a full listing of topics. MOST of them are here, but some could be missing. I personally think this sheet is fairly helpful in that it is yet another stating of the topics we covered - some of this might even make it into the eBook eventually. - Dr. McCordElectronegativity and Polarity
Memorize the trend in electronegativity and that fluorine is the top-dog at 4.0 and oxygen comes in second at about 3.5. Note that all the non-metals are greater or equal to 2.0 while the metals fall below that (ok, most of the metals). This is a relative measure of the “PULL” on electrons. Unequal pulling on electrons and where they are ultimately determine the polarity of molecules. If we only look at bonds we can quickly assign \(\delta^+\) and \(\delta^-\) (read that as partial positive and partial negative) to the two ends by looking up the electronegativities. However, a polar bond does not mean that the entire molecule is polar. You must consider the overall geometry and symmetry. You must also look at lone pair electrons in the molecule. A polar molecule MUST have a non-zero net dipole moment. This means that you must add up all the individual polar bond dipoles and see if they all cancel out or do you get an overall dipole. All five of the basic electronic geometries we’ve covered are perfectly symmetric as long as ALL the positions are equivalent. Any mismatch (loss of symmetry) will result in a polar molecule. Lone pairs are a dead give away for polarity. The only match to cancel a lone pair is another lone pair 180° opposite or 2 lone pairs each 120˚ away (as in the trigonal planar equatorial positions in the trigonal bipyramidal geometry). This is only possible on a couple of our molecular shapes – look and see which ones on the help sheet.
Dipole Moments and Polarity
When there is a net dipole moment for a polar molecule it can be measured. The strength of a dipole is a measurement of its dipole moment. A quantity of charge (\(q\)) is separated by a distance (\(r\)) and the dipole moment is easily shown mathematically to be:
$$ \mu = q \cdot r $$
You can read more about this from other online sources. Make sure you know this though: ANY polar molecule MUST have a non-zero net dipole moment. All non-polar molecules will have zero net dipole moments. Remember that symmetry is the biggest factor you must consider when assigning whether a molecule is polar or not. Symmetry will mathematically cancel out polar bond vectors within a molecule.
Bond Lengths and Strengths
Know the trends in bond strengths. How does a single bond relate in strength to a double bond? Triple bond? How about Bond lengths? Take a peak at the Table of Bond Energies in our eBook. The obvious conclusion here is that triple bonds are the strongest, followed by double bonds, and then single bonds. That is the TREND. And, while we're at it, that strong triple bond tends to PULL the nuclei closer together and therefore tends to make triple bonds shorter in length that the corresponding double and single. The same goes for a double bond being shorter than the corresponding single.
VSEPR and VB Theories
Know what each of these theories “brings to the table” as far as chemical bonding and shapes goes. While I'm at it - you DO know what VSEPR and VB stand for - right? You’ll need to know all the shapes that are shown in our eBook – linear to octahedral which matches up with 2 electron regions through 6 electron regions. Know the VB hybrids that are necessary to get each shape that we covered - VSEPR will help you arrive at the right shape but it says nothing about HOW you get there. VB theory steps in and helps us visualize how this gets done. By allowing atomic orbitals to hybridize, we can get all the necessary geometries and we can “see” orbitals on neighboring atoms overlapping to give bonding orbitals (\(\sigma\) and \(\pi\) bonds). Know your shapes, angles, angle tweaks, and hybridization. Be sure and check out my other help sheet on VSEPR and VB Theory - and read the book.
One more thing...If someone asks what the "geometry" is of a molecule they are referring to the molecular geometry. One has to specify electronic geometry if that is what they mean - molecular geometry is always implied.
Angle “Tweaking”
Yes, of course you must know the standard bond angles for the five electronic geometries. You must also know how to “tweak” a bond angle based on the slight differences in electron region repulsions. We say that lone pair electrons are more repulsive than any bonding pair. This means that lone pairs need a bit more space and therefore “squish” the bond angles that are adjacent to it. The "squish" amount varies, but is typically around 2-2.5° per lone pair (or LESS). You generally don't tweak beyond about 5° max. So keep this in mind if you are trying to chose the best choice in a question.
The fact that lone pairs are more repulsive than bonding pairs is also why lone pairs always go into the equatorial positions of a trigonal bipyramid electronic geometry and never go into the axial positions. There is more "room" in the equatorial positions.
Sigma (\(\sigma\)) and Pi (\(\pi\)) Bonding
VB theory (LE or Localized Electron theory in some books) also brings us \(\sigma\) and \(\pi\) bonds. Know the key difference in these two types of bonds. What kind of overlap does each bond use? Which one is generally stronger? A double bond in VB theory is always a \(\sigma\) bond reinforced with a \(\pi\) bond. A triple bond is a \(\sigma\) bond reinforced with two \(\pi\) bonds. Unfortunately VB theory doesn’t always work out – especially for certain non-bonding electrons and radical species (O2 is a good example).
Interpreting LINE Structures of Organic Molecules
We had plenty of "practice" in class via clicker questions on interpreting line structures. Make sure you can analyze the structure and know how many carbons and hydrogens are implied in the structure. Also know the angles and hybrids of each and every atom in the structure. Always assume there are carbons at all line intersections and bends in the structure - also at the end point if nothing is shown there. Make sure each carbon in the structure has 4 total bonds (lines) to it. All "missing" bonds are assumed to be H's in the structure. ALL non-carbon and non-hydrogen atoms will be SHOWN. You will also have to provide any missing lone pairs in the structure. The animated picture below shows the original line structure (black), then the carbons you interpret (green), and then the hydrogens and lone pairs you interpret (red).
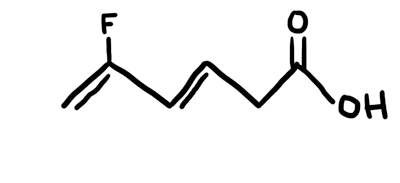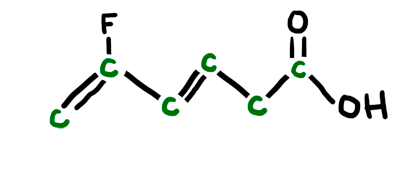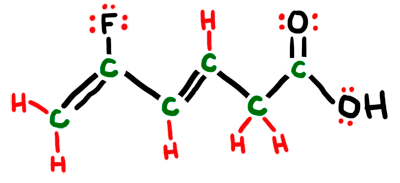
MO Theory
In MO theory we use the atomic orbital wavefunctions (\(\psi\)) to generate new molecular orbital wavefunctions for the electrons in the complete molecule. For each atomic orbital we will get a new molecular orbital. MO theory still uses the σ and π designations for bonding orbitals. MO also introduces antibonding orbitals \(\sigma\)* and \(\pi\)* (sigma-star and pi-star). Antibonding orbitals are the result of destructive interference when combining the wavefunctions for the atomic orbitals (\(\psi_1\) and \(\psi_2\) are out of phase). Know the relative energies of bonding vs antibonding orbitals. Know the ordering of the molecular orbitals for all 2nd row homonuclear diatomic molecules. Know HOW to fill this diagram properly with electrons. For heteronuclear diatomics know how to fill in a diagram if one is given to you. You should also realize that the MO diagram for heteronuclear molecules with atomic orbitals of similar energy (such as NO) are essentially the same as the homonuclear diatomics. In heteronuclear diatomics the more electronegative element will have the lower orbital energies. As a result, the bonding MOs will more closely resemble the atomic orbitals from those elements. The anti-bonding will be more similar to the atom with the smaller electronegativity (aka: the more electropositive one).
And finally, there are times in which atomic orbitals (AO) on one atom don’t overlap at all with the AOs on the other atom. This results in non-bonding orbitals which is where lone pair electrons tend to reside. These orbitals are essentially identical to the AO on that element. They don’t lower or raise the energy compared to the AO. Therefore they don’t participate in the bonding (or bond order calculation).
Bond Order - MO Theory
Bond order (BO) was defined and used back in chapter 13 when discussing Lewis structures. You basically counted the number of lines in a bond and had your answer: triple bonds have 3.0 for BO, double bonds have 2.0, and single bonds have 1.0. Resonance structures allowed use to get fractional bond orders by showing multiple structures where the \(\pi\)-bond was delocalized.
MO Theory makes calculating BO even more straight forward. BO is calculated from the simple formula:
\[{\rm bond\;order} = {{{\rm (\#\;\;bonding\;\;e^-)} - {\rm (\#\;\; antibonding\;\; e^-)}}\over 2} \]
The key here is filling the MO diagram correctly so that you have the right number of each type of electron. Also, when there are multiple atoms in the molecule, this formula gives the overall bonding order for the entire molecule - not just for one bond.
Orbitals in Polyatomic Molecules
Know what the overall concept is. We often COMBINE VB theory and MO theory taking the best parts out of each. Don’t fret the details but do know that atomic orbitals will combine in all sorts of ways to get you various levels of energies within a molecule. Check out how the \(\pi\)-system in benzene is really represented with MO’s even though the 6-carbon ring is best pictured as 6 carbon atoms with sp2 hybridization. Realize that the idea of bond “delocalization” is best understood from the molecular orbital model. I DID spend plenty of class time on the concept of \(\pi\)-systems. Know what that is and how it is manifested in a molecule.
Diamagnetism vs Paramagnetism
What’s the difference in diamagnetic and paramagnetic? Diamagnetic is where all electrons are paired within a molecule (↿⇂). Paramagnetic is when you have one or more unpaired electrons (↿ ) in a molecule. MO Theory properly predicts (unlike the Lewis dot structure) the paramagnetic nature of oxygen, O2.
Chapter 16 - Liquids and Solids
Why molecules stick
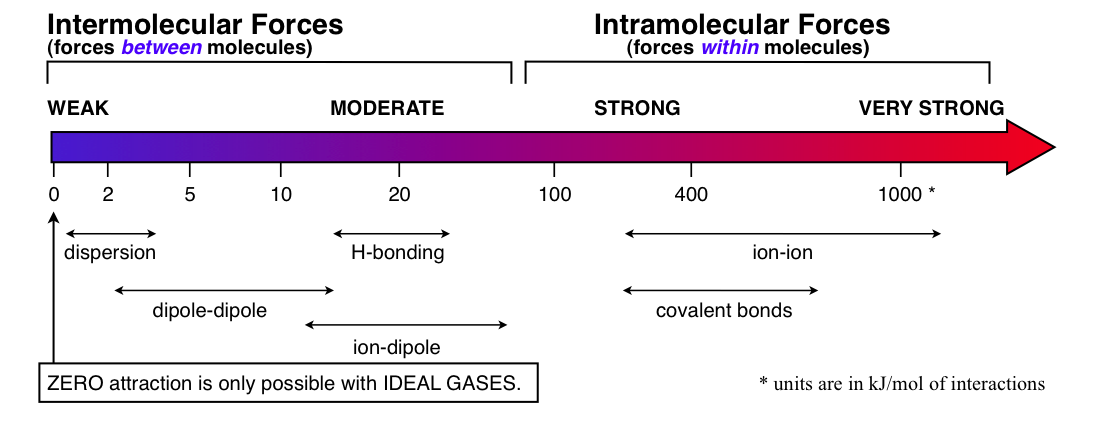
If molecules had no sticking power, all substances would be gases. There would be no condensed phases of solid or liquid. Well thank goodness there ARE forces of attraction (the sticky) between molecules. These forces are called intermolecular forces (IMFs). They are also known as Van der Waal’s forces. These are the forces that govern various physical properties such as boiling point, melting point, surface tension, viscosity, etc… I’ll come back to this after I remind you of the forces of attraction that we have all ready covered quite extensively.
Intramolecular forces
Intramolecular forces are the forces of attraction within molecules and are simply the bonds that hold the atoms together to make the molecule in the first place. These are considerably large forces. Have yet another look at the Table of Bond Energies in our eBook. You can see just how strong an intramolecular force (covalent bonding) really is. Those are the amounts of energy that would be required to pull those bonds apart which is why they are all positive and not negative values of energy. The lowest value on that table is 149 kJ/mol and goes up to 1072 kJ/mol for a carbon-oxygen triple bond. Realize that is just a sampling of all the possible covalent bond strengths, but you need to have an idea of where all those strengths are on the energy scale and what their average is. Let’s put the average at around 400 kJ/mol. I also like to include ionic bonding here which is what holds ionic compounds (salts) together. They too are very strong forces reaching as high as 15,000 kJ/mol for some 3+ to 3- salts. So all of those were BIG FORCES. Now let’s get back to the main topic here...
Intermolecular forces (IMFs)
First, DO realize that intermolecular forces are much much less than intramolecular forces. On average, intermolecular forces are 100 times less depending on what you are comparing. All intermolecular forces are governed by partial charges attracting or repulsing one another. Heck, pretty much everything chemically speaking can be traced back to this fundamental interaction of charges. Bearing that in mind, it is still not just a simple case of positive attracts negative. We want to quantify the amount of attraction/repulsion so that we can better understand and predict the properties that are a result of these intermolecular interactions.
What’s in a name? When its Van der Waals, its a lot.
Intermolecular Forces have historically also been called Van der Waal forces which is really the correct interpretation - meaning it covers all the forces between molecules. However, be warned, many books (and questions) equate Van der Waal forces with ONLY dispersion forces. Check out Wikipedia for Van der Waals forces - you’ll see there under the definition section that the term can be meant one way or another. Best to know the authors intent as they say. Unfortunately our history is steeped in tradition and the term on Quest usually refers to just dispersion forces. We’ll try to stick with this concept on the exam. In other classes (biology, etc...) you might have to adjust your thinking on this term.
Ion-Ion interaction
As I mentioned before, this force is a big one. It really isn't a true "intermolecular" force because ionic compounds aren't molecules. Cations and anions combine to make ionic compounds or salts which are technically NOT molecules so the whole “molecular” term doesn’t really fit. None-the-less, ion-ion interaction IS the reason we have ionic compounds. All ionic compounds are solids because of this great big pull that cations have on anions (and vice versa). Ion-ion interaction is what gives us the large values for the crystal lattice energy of salts. The potential energy (\(E_P\)) of this interaction is:
$$E_{\rm P} \propto {{q_1q_2}\over r}$$
Where \(q_1\) and \(q_2\) are the charges on the two ions and \(r\) is the distance between them. It’s worth noting that when oppositely charged ions are involved (positive/negative), the potential energy is lowered (negative values) due to the attractive forces. Positive potentials results when like charges come together (repulsions). Also, when highly charged ions come together (like +3 and -2) you can get lattice energies well over 10,000 kJ/mol.
Dipole-dipole interaction
We now knock down the strength of the positive/negative interaction by a considerable amount. Now we use partial positive and partial negative as our “sticking points”. Dipoles truly deserve the word partial in partial charge. Only a little bit of charge is there - a little positive (\(\delta^+\)) and a little negative (\(\delta^-\)). Although there is variability in the amounts of these charges (think electronegativity scale), they are still piddly little compared to full-blown charge found in cations and anions. This interaction in equation form is shown as
$$E_{\rm P} \propto -{{\mu_1\mu_2}\over r^3}$$
Where \(\mu \) is the dipole strength (dipole moment). Notice that the distance dependence is \(1/r^3\). From distance alone this puts the force at about 1% of ion-ion attraction. Most "regular" dipole-dipole potential energies have averages around 4 to 5 kJ/mol of interactions. I say "regular" because I must contrast this to H-bonding which is just a stronger version of the same thing. Also remember that these dipoles are permanent dipoles and are at the heart of polar substances.
H-bonding
When a H atom is covalently bonded to a N, O, or F atom, a very polar bond is formed. This bond is so polar that the H atom is almost stripped completely of its electrons. To the outside world (just outside that molecule mind you) that H atom looks almost like a bare proton. It carries a very large partial positive charge – more so than any other partial positive that you might encounter. This H will then act like a magnet for the lone pairs of electrons on its neighboring molecules. In particular, it goes after the lone pairs on the other H-bonded N’s , O’s , and F’s. The interaction is strong enough to get it the name H-bonding. Bear in mind that the interaction is NOT an actual covalent bond, but it is about 10% of the strength of a covalent bond and that is why it gets that name. If a typical H-bond dipole is double a "regular" dipole, then you can see how the result from the equation above would be about 4 times the \(E_P\) value. For this reason, the average H-bond interaction has a potential energy of around 20 kJ/mol of interactions. Water is up around 36 kJ/mol. Note that H-bonding doesn’t have a different equation than dipole-dipole. They are both the same equation - it’s just that the dipoles are considerably bigger for H-bond type substances.
(London) Dispersion Forces
Sometimes the word "dispersion" is not there, and sometimes the word "London" is not there. Sometimes the term Van der Waal’s Forces are (mistakingly) used to describe only these particular forces. The fact is that dispersion forces are present in ALL molecules, polar AND non-polar. The roll they play (whether major or minor) is what we must consider. These forces are the ONLY force for all non-polar molecules. All substances CAN be liquefied and therefore they ALL have some degree of attractive forces within them. The potential energy for London dispersion forces is
$$E_{\rm P} \propto -{{\alpha_1\alpha_2}\over r^6}$$
Where \(\alpha \) is the polarizability of the molecules. The polarizability quantifies the tendency of a collection of charges (like the electrons in a molecule) to become distorted by an applied electric field (like a nearby charge or dipole). The larger the molecule, the more polarizable and therefore, the greater the London forces. For atomic systems, the higher the atomic number the larger the polarizability and thus the London force. This is because the more electrons there are the more they start to populate the larger atomic orbitals. The further out an electron is from the nucleus the easier it can be influenced and moved by another source. Once the charge is shifted you get a temporary dipole - there one minute, gone the next (or should I say femtosecond?). Temporary dipoles are the heart of dispersion forces - these are also known as instantaneous dipoles in some books.
Once you have the temporary dipole, you can have it participate in a dipole-induced dipole. This is the result when a non-polar (no permanent dipole) molecule can be induced to have a dipole. It doesn’t matter if it is induced by a real (permanent) dipole from a polar molecule or whether by a temporary dipole from a non-polar molecule. Once set-up, the partial charges in the dipoles do cause attractions. These attractions are reinforced, the bigger the molecule happens to be.
Now if we only look at that the \(1/r^6\) part of that equation we can certainly see why dispersion forces are the smallest of all intermolecular forces. At the same distances, a dispersion interaction can be 1000 to 10000 times less than ion-ion interactions. That is so small, that if there is no appreciable polarizability (\(\alpha\)), then the substance is most likely a gas at normal temperatures (case in point : all the noble gases). So by taking into account distance (\(r\)) and typical \(\alpha\)’s we find the typical molecule to have about 2 kJ/mol for this interaction - certainly the smallest of our three intermolecular forces. But hey! That 2 kJ/mol average is only for a "typical" little molecule. What exactly IS a typical molecule? That’s a loaded question if ever there was one. Let’s try this in a different way, read on...
Relatively small molecules are going to have very small amounts of dispersion forces present. As a matter of fact, very small molecules will almost always be gases because of this. Water is one of those rare small molecules that has enough "pull" to become a liquid at room temperature thanks to H-bonding between the molecules. As molecules get larger (careful, larger in size doesn’t always mean mass - remember your trends), polarizability increases and dispersion forces start playing a larger role in the cohesiveness (see cohesive forces later in this review) of a substance. Two molecules could have the same mass and make-up (isomers) and the one with the largest surface area will have the largest dispersion forces. Increasing surface area will increase the number of electrons available for polarization and therefore increase the forces in between.
Ion-dipole interaction
Now we are cross-matching force types! You got half of the strong interaction of ion-ion but you’ve lost the other half to a mere dipole. The equation you get is a combination of the ion-ion one and the dipole-dipole one.
$$E_{\rm P} \propto -{{|q|\mu}\over r^2}$$
Where \(q\) is the charge on the ion (+ or – doesn’t matter) and \(\mu\) is the dipole strength. Note the \(1/r^2\) dependence - this pulls this force up into the same league as H-bonding and a little extra (depends on the ion). It’s a little misleading also in that you must have both ions and dipoles for this to exist. You get it 2 ways: (1) Dissolve a salt into water and you’ve got ions swimming amidst dipoles. (2) Or you can have a salt (ions) that “pulls” some polar molecule (dipole, probably water) into its crystal lattice. This is precisely what happens with “hydrate” salts. If you have an ion with a high charge density (charge/size), it will greatly attract water molecules via this interaction. So much so that the salt will form with water molecules in the salt. Sodium carbonate has a Na+ ion which is small enough to pull in a water - actually 10 waters. The new formula is Na2CO3·10H2O. That is sodium carbonate decahydrate. The waters of hydration are shown separately from the salt but attached via the dot. Realize that dot is just an attachment and NOT a multiplication. CuSO4·5H2O is another example of a hydrated salt. These waters are strongly attached and can only be driven off via high temperatures (much more than 100°C). Remember, the higher the charge, the more likely this will happen - most +2 and +3 cations will pull in waters. Li+ and Na+ are the only +1 cations that are small enough to still pull in the water, all the other group 1A cations do not form hydrates.
Get that Intermolecular Feeling...
Now that we’ve got all those different types of interactions down lets look at the figure at the end of this review sheet and get a feel for the actual amounts of energy involved. Look at it and realize that those are approximate values. Take a look at my figure and try to get a good mental picture of all these interactions and their relative values. Are you feeling it? I knew you could. I must remind you that although dispersion forces are the weakest of all the forces, that is just per single interaction. Real molecules have real shapes, volumes, and sizes. Those little interactions can certainly add up to a substantial amount of force. It is for this reason that most substances are solids once you get a big enough molecule. The dispersion forces are quite impressive in great numbers.
A Diagram for your Perusal
Do realize in the following diagram that dispersion forces are capable of much more when the molecule (or atom) containing them increases in size. Polarizability will increase considerably with a molecule’s surface area (size). One should always access what the conditions are as to which force is the governing force and what its magnitude is. (This diagram is available as a pdf on our Help Sheets page.)
Put it all together now
You now know the forces that hold substances together. You also know the relative strengths of those forces. So now you should be able to predict some relative outcomes of physical properties DUE to those forces.
So read the book and your notes (and any other reference you want) and KNOW how the following properties are affected by intermolecular forces.
viscosity
surface tensioncapillary action
meniscus (shape?)vapor pressure
melting and boiling point
Think about what the property does when forces get bigger and smaller. How does temperature affect these forces? BTW, nobody should miss how temperature affects these forces. You DO know right? The hotter you get something, the more likely you are to evaporate or melt it, right? The end result of constant heating is to vaporize everything into gas state – which happens to be the state where ALL these forces of attraction are so RELATIVELY weak that they can be ignored. The thermal motion brought on by heat will eventually overcome any “sticking power” that is present. More heat, less stick, less stick, less likely to be a solid or liquid and MORE likely to become a gas. See, that wasn’t too hard was it. Hopefully this makes perfectly logical sense to you. And the truer that previous sentence becomes, the better your understanding will be of the material.
Adhesive vs Cohesive Forces
Adhesive forces are the forces of attraction between a liquid and a surface (think water-to-glass). Cohesive forces are the forces of attraction between a liquid and itself (think water-to-water). Both of these forces are actually rooted in the same intermolecular forces that we have already discussed. When the adhesive forces are greater than the cohesive forces, a liquid will tend to try and wet the surface. The liquid will MAXIMIZE attractive forces by maximizing the contact with the surface (see NOTE below). This is what happens with water on glass. The water tends to “crawl up” the side of a glass wall and maximize contact.
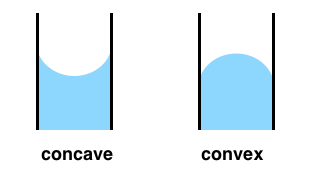
If you have a LARGE glass container, you hardly notice the water that has crawled up the wall. Overall, the water does look pretty flat. However, if you force the surface area of the water to be smaller and smaller by shrinking the diameter of the glass container, you will start to SEE this crawling up AND the surface will start to look concave. This new spherical shape on the surface is called a meniscus. The fact that there is a meniscus has everything to do with surface tension also. If you have no surface tension (or close to none), you will have no meniscus.
Read More and Read Often
Once again I’m asking you to READ. Yes, read our ebook, read this, and find other sources to read. Whatever you read, read it in context. Remember that there is a Chemistry Library in Welch that has lots of general chemistry textbooks for you to peruse.