Question 10.5 pts
Consider the reaction:
2O3(g) 3O2(g) rate = k[O3]2[O2]-1
What is the overall order of the reaction and the order with respect to [O3]?
Question 20.5 pts
When the reaction below:
3NO(g) N2O(g) + NO2(g)
is proceeding under conditions such that 0.015 mol/L of N2O is being formed each second, the rate of the overall reaction is ________ and the rate of change for NO is ________.
Question 31.0 pts
What is the rate law for the reaction below:
A + B + C D
if the following data were collected?
| Exp | [A]0 | [B]0 | [C]0 | Initial Rate |
| 1 | 0.4 | 1.2 | 0.7 | 2.32x10-3 |
| 2 | 1.3 | 1.2 | 0.9 | 7.54x10-3 |
| 3 | 0.4 | 4.1 | 0.8 | 9.25x10-2 |
| 4 | 1.3 | 1.2 | 0.2 | 7.54x10-3 |
Question 41.0 pts
A chemical reaction is expressed by the balanced chemical equation:
A + 2B C
Consider the data below:
| exp | [A]0 | [B]0 | initial rate (M/min) |
| 1 | 0.15 | 0.15 | 0.00110363 |
| 2 | 0.15 | 0.3 | 0.0044145 |
| 3 | 0.3 | 0.3 | 0.008829 |
Find the rate law for the reaction.
Question 50.5 pts
Question 60.5 pts
Question 71.0 pts
We know that the rate expression for the reaction below:
2NO + O2 2NO2
at a certain temperature is rate = [NO]2 [O2]. We carry out two experiments involving this reaction at the same temperature, but in the second experiment the initial concentration of NO is doubled while the initial concentration of O2 is halved. The initial rate in the second experiment will be how many times that of the first?
Question 81.0 pts
Consider the data collected for a chemical reaction between compounds A and B that is first order in A and first order in B:
| rxn | [A]0 | [B]0 | rate (M/s) |
| 1 | 0.2 | 0.05 | 0.1 |
| 2 | ? | 0.05 | 0.4 |
| 3 | 0.4 | ? | 0.8 |
From the information above for 3 experiments, determine the missing concentrations of A and B. Answers should be in the order [A] then [B].
Question 90.5 pts
Question 100.5 pts
Consider the reaction below:
A + B C
If it is 1st order in A and 0th order in B, a plot of ln[A] vs time will have a slope that is...
Question 111.0 pts
Consider the reaction below:
H2CO3(aq) CO2(aq) + H2O(l)
If it has a half-life of 1.6 sec, how long will it take a system with [H2CO3]0 of 2M to reach [H2CO3] of 125mM?
Question 121.0 pts
At a certain fixed temperature, the reaction below:
A(g) + 2B(g) AB2(g)
is found to be first order in the concentration of A and zeroth order in the concentration of B. The reaction rate constant is 0.05s-1. If 2.00 moles of A and 4.00 moles of B are placed in a 1.00 liter container, how many seconds will elapse before the concentration of A has fallen to 0.30 moles/liter?
Question 130.5 pts
The reaction below:
A products
is observed to obey first-order kinetics. Which of the following plots should give a straight line?
Question 141.0 pts
For the reaction below:
cyclobutane(g) 2ethylene(g)
at 800K, a plot of ln[cyclobutane] vs t gives a straight line with a slope of -1.6 s-1. Calculate the time needed for the concentration of cyclobutane to fall to 1/16 of its initial value.
Question 151.0 pts
Question 161.0 pts
Question 170.5 pts
Consider the following elementary reactions:
a) NO + O3 NO2 + O2
b) CS2 CS + S
c) O + O2 + N2 O3 + N2
Identify the molecularity of each reaction respectively.
Question 181.0 pts
A and B react to form C according to the single step reaction below:
A + 2B C
Which of the following is the correct rate equation for [B] and the correct units for the rate constant of this reaction?
Question 191.0 pts
Consider the mechanism below:
NO2 + F2 NO2F + F k1, slow
F + NO2 NO2F k2, fast
What is the rate law?
Question 201.0 pts
Determine the overall balanced equation for a reaction having the following proposed mechanism:
Step 1: B2 + B2 E3 + D slow
Step 2: E3 + A B2 + C2 fast
and write an acceptable rate law.
Question 211.0 pts
Consider the reaction below:
H2(g) + I2(g) 2HI(g)
The proposed mechanism of this reaction is:
I2 ⇌ 2I k1, k-1(reverse rxn), fast
2I + H2 2HI k2, slow
What is the rate of the overall reaction?
Question 221.0 pts
Question 231.0 pts
Question 241.0 pts
For the reaction below:
HO(g) + H2(g) H2O(g) + H(g)
a plot of lnK vs 1/T gives a straight line with a slope equal to -5.1x103 K. What is the activation energy for this reaction?
Question 251.0 pts
Question 261.0 pts
Question 271.0 pts
Question 281.0 pts
Question 291.0 pts
Consider the potential energy diagram below:
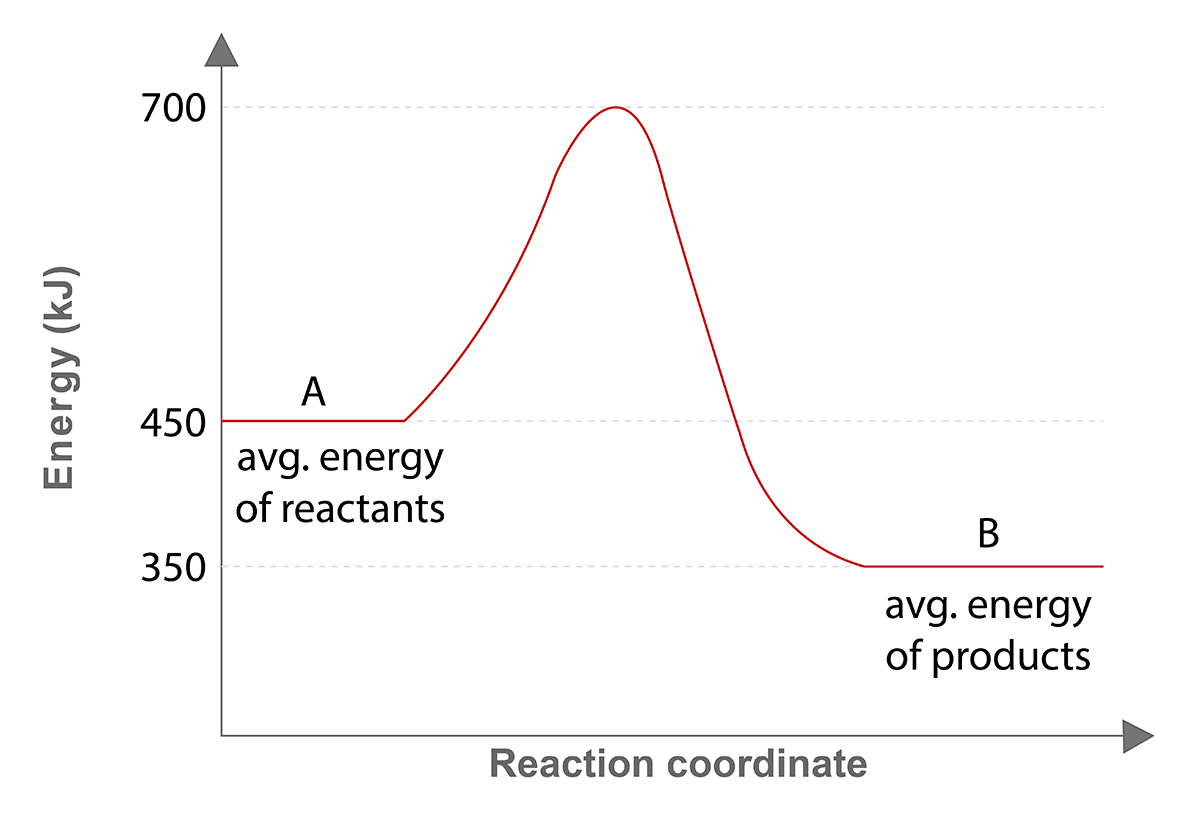
What is the change in enthalpy () for the reaction A
B?
Question 301.0 pts
Question 311.0 pts
Question 321.0 pts
"Reaction mechanisms usually involve only unimolecular or bimolecular steps."
Is this statement true or false?
Question 331.0 pts
Which of the following is/are ALWAYS true concerning collision and transition state theory?
I) Transition states are short-lived.
II) A balanced reaction shows which species must collide for the reaction to occur.
III) Intermediates are short-lived.
Question 341.0 pts
Consider the following reaction mechanism:
1) Cl2 + Pt 2Cl + Pt
2) Cl + CO + Pt ClCO + Pt
3) Cl + ClCO Cl2CO
Overall: Cl2 + CO Cl2CO
Which species is/are intermediates?
CH302 · 50520 Principles of Chemistry II
Spring 2023 · © mccord