Question 11.5 pts
Use the phase diagram for CO2 provided below to answer the following question:
At 300K and 10 bar, what is the stable phase of carbon dioxide?
Question 21.5 pts
Use the phase diagram for CO2 in the question above to answer the following:
A sample of carbon dioxide is stored at 10,000 bar and 250K. This sample is then decompressed to 1 bar at constant temperature. Then, at constant pressure it is heated to 400K. Next, it is compressed at constant temperature to 200 bar. According to the phase diagram, how many phase changes has the sample of carbon dioxide gone through, and what is its final state?
Question 31.5 pts
Question 41.5 pts
Question 51.5 pts
Question 61.5 pts
Question 71.5 pts
Which of the following would increase the solubility of a gas in water?
1. increase the temperature of the water
2. decrease the temperature of the water
3. increase the pressure of the gas above the water
Question 81.5 pts
Question 91.5 pts
Question 101.5 pts
Question 111.5 pts
Question 121.5 pts
Substances A and B are mildly volatile solvents. Using the diagram below, determine the mole fraction of B when the vapor pressure of the mixture is 80 Torr.
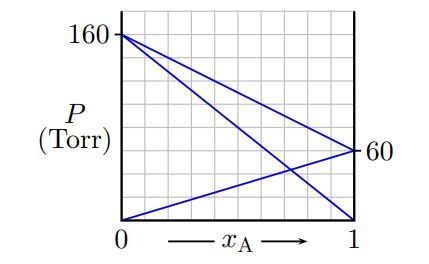
Question 131.5 pts
Question 141.5 pts
Question 151.5 pts
What will be the freezing point of a solution of 8 moles of sodium dichromate (Na2Cr2O7) dissolved in 16 kg of water? Please answer in K and round to the first decimal place.
Use the following values:
Kb = 0.512 K/m
Kf = 1.86 K/m
Question 161.5 pts
Question 171.5 pts
Question 181.5 pts
Question 191.5 pts
Two aqueous solutions are separated by a semi-permeable membrane:
Solution A = 0.34 M KCl
Solution B = 0.34 M MgCl2
Which of the following statements is TRUE?
Question 201.5 pts