Wednesday 2/9
9:00am - 9:50am
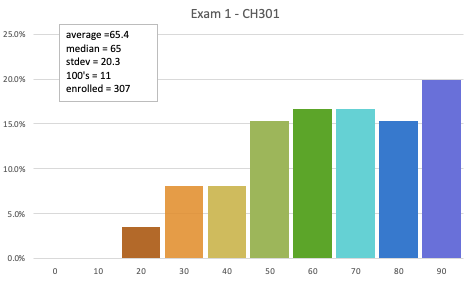
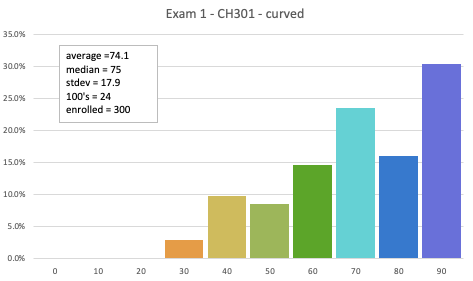
Coverage: Exam 1 covers all the material that was covered on LE's 01-09 and HW01, HW02, and HW03. Most of the exam is over Chapter 2 (Gases) from the gchem site. There will be about 3 questions that are "fundamentals" (Chapter 1) which means you would be balancing and equation and calculating stoichiometric quantities (especially gases). Know that this exam is really about gases.
About ChemBook Those chapters are listed as a reference. You are NOT being tested on ChemBook content - so don't email me and ask. But if you'd like some nice tie-ins to the real world, then read some chembook stuff. Many former CH301 and CH302 students have pointed out how much they liked chembook as an extra reference. Plus, some of the chembook pages and info is presented better than in gchem which is why I will often reference from chembook. Feel free to dig into OpenStax as well if you want. It's all the same chemistry. 🙂
Students will know...
Students will be able to...
Jimmy's Help Videos on Exam 1 topics.
\(PV=nRT\)
Boyle's Law: \(P_1V_1 = P_2V_2\) (constant n, T)
Charles' Law: \(\displaystyle{V_1\over T_1} = {V_2\over T_2}\) (constant n, P)
Avogadro's Law: \(\displaystyle{V_1\over n_1} = {V_2\over n_2}\) (constant P, T)
Gay-Lussac's Law: \(\displaystyle{P_1\over T_1} = {P_2\over T_2}\) (constant n, V)
Inflate a Tire Law: \(\displaystyle{P_1\over n_1} = {P_2\over n_2}\) (constant V, T)
Nobody Does This Law: \(n_1T_1 = n_2T_2\) (constant P, V)
(note that you will be tested on the actual relationships of the physical properties and not the scientist's names)
and because density (ρ) is m/V and molar mass (M) is m/n, then you also know the molar mass of an ideal gas via:
\[{M={\rho RT\over P}}\]
Dalton's Law of Partial Pressures
\[P_{\rm total}=P_{\rm A} + P_{\rm B} + P_{\rm C} + \cdots\]
mole fraction of gas A: \(x_{\rm A}=P_{\rm A}/P_{\rm total}\)
Dalton's Law of Partial Pressures: \[P_{\rm total}= P_{\rm A} + P_{\rm B} + P_{\rm C} + \cdots \]
Kinetic Energy of an Ideal Gas: \[E_{\rm k} = {3\over 2} RT\]
Root Mean Squared Speed of particles: \[v_{\rm rms} = \sqrt{3RT\over M}\]
Van der Waal's Equation of State
\[\left(P+a{n^2 \over V^2}\right)(V-nb)=nRT\]
Here are two practice problem sets for you to work on and get some experience working through these types of problems.
Composition Stoichiometry Extra Practice.pdf HTML version
Gas Law Stoichiometry Extra Practice.pdf HTML version
Sorry, but we do not have any answer keys to all these problem. The HTML version of the Gas Stoich does have answers. Talk with other students about the problems and try to reach a correct conclusion/answer.
There is even MORE great practice problems (with keys) and helpsheets on the gchem site in Chapter 1 there under "Additional Content > Helpsheets and Worksheets". Here's a direct LINK to that page.