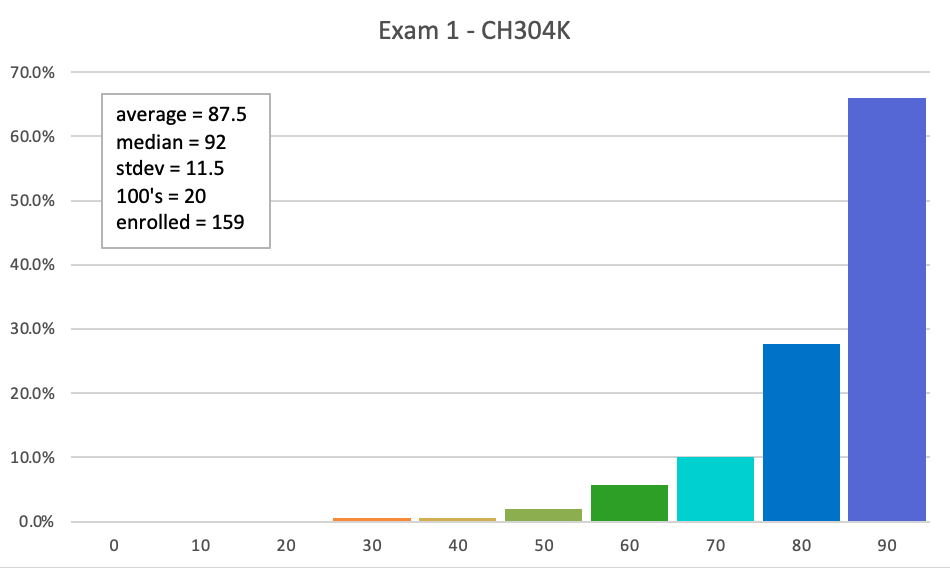
Exam 1
Thursday 2/11
12:30-2pm
Learning Outcomes
Students will know...
- how to count stuff
- that fire is hot
- how to mathematically convert from one type of unit to another utilizing a set of conversion factors
- the names, formulas, and physical state of the first 10 alkanes
- Know which elements exist as diatomic molecules
- the MAIN Metric Prefixes for Chemistry Class as listed in section 10.2 of chembook - it's the last table there
- how to fully balance a chemical reaction and identify the coefficients
- how to do composition stoichiometry calculations - figuring out the percent of a specific element in a given compound
- how to do reaction stoichiometry calculations converting moles to moles and also moles to grams and grams to grams or anything else
- how to predict product amounts when given arbitrary amounts of reactants - limiting reactant problems (like #20 on HW01)
- the same outcomes as the two previous ones but with gas moles using the ideal gas law to get pressure or volume of the gas reactants.
- the 3 primary components and their percentages of dry air
- how those percentages change when humid air is used
- the 6 primary pollutants in our air - know names and formulas and/or abbreviations for them
- the primary sources/causes of those pollutants
- what methods are in place to help curb the amounts of these pollutants in air
- how to calculate various gas law values - P, V, T, and n according to the ideal gas law and associated laws
- how to convert pressure of a gas into number (mole) density
- what partial pressure is and how to calculate it.
- how to get mole fraction from partial pressure and total pressure and vice versa
- how to use the pressure and identity of a gas to calculate its mass density
- how to convert mass density and pressure into the molecular weight of a gas
- anything else we learned and did in class, on HW, that I forgot here
More Help on Exam 1
Jimmy's Help Videos on Exam 1 topics.
Formulas YOU should know
The Ideal Gas Law (IGL):
\(PV=nRT\)
Gas Laws "contained" in the IGL:
Boyle's Law: \(P_1V_1 = P_2V_2\) (constant n, T)
Charles' Law: \(\displaystyle{V_1\over T_1} = {V_2\over T_2}\) (constant n, P)
Avogadro's Law: \(\displaystyle{V_1\over n_1} = {V_2\over n_2}\) (constant P, T)
Gay-Lussac's Law: \(\displaystyle{P_1\over T_1} = {P_2\over T_2}\) (constant n, V)
Inflate a Tire Law: \(\displaystyle{P_1\over n_1} = {P_2\over n_2}\) (constant V, T)
Nobody Does This Law: \(n_1T_1 = n_2T_2\) (constant P, V)
(note that you will be tested on the actual relationships of the physical properties and not the scientist's names)
and because density (ρ) is m/V and molar mass (M) is m/n, then you also know the molar mass of an ideal gas via:
\[{M={\rho RT\over P}}\]
Dalton's Law of Partial Pressures
\[P_{\rm total}=P_{\rm A} + P_{\rm B} + P_{\rm C} + \cdots\]
mole fraction of gas A: \(x_{\rm A}=P_{\rm A}/P_{\rm total}\)
Practice Problem Sets
Here are two practice problem sets for you to work on and get some experience working through these types of problems.
Composition Stoichiometry Extra Practice.pdf
Gas Law Stoichiometry Extra Practice.pdf
Sorry, but we do not have any answer keys to these problem. Talk with other students about the problems and try to reach a correct conclusion/answer.
Exam protocol All you need to know about HOW to take our online exams.