McClicker Questions for the Semester - if you don't know the answer, go to the class notes for that day and find the McClicker question.
Order is now in REVERSE. Most recent at the top.
Tue 11/29
SCENE: You have a glass of water in your left hand and a spoon full of table salt in your left hand. Now you stir the salt into the glass of water and stir. As the salt dissolves, you sense what? Hint: Dissolving salt in water is an endothermic process.
- You notice a slight cooling in the glass of water.
- You notice a slight warming in the glass of water.
Tue 11/15
This is almost identical to #7 on HW08...
Using bond energies (chembook 10.7), calculate the heat of combustion for propane. This is the amount of heat that is released. Answer in kJ/mol of propane.
Hints: first write out the balanced equation for the combustion of 1 mole of propane. You will BREAK all the bonds in all the reactants. You will MAKE all the bonds in the products. Do the math.
- 2012 kJ/mol
- 6486 kJ/mol
- 8498 kJ/mol
- 2220 kJ/mol
- 14984 kJ/mol
Thu 11/10
Which of the following hydrocarbons is the most likely product formed from cracking C18H38?
- CH4
- C3H8
- C36H74
- C9H20
- CO2
Tue 11/08
Calculate the approximate \(\Delta H\) of this substitution reaction using bond energies.
- –624 kJ/mol
- +624 kJ/mol
- –54 kJ/mol
- +54 kJ/mol
- –7 kJ/mol
- +7 kJ/mol
Thu 11/03
A liquid fuel releases 35 kJ/g when burned. The density of this fuel is 0.82 g/mL. How much energy is released when 5.5 mL of this fuel is burned?
- 121 kJ
- 168 kJ
- 235 kJ
- 193 kJ
- 158 kJ
Tue 11/01
I have two metals to compare. Metal-X which has a specific heat capacity of 0.36 J/g °C, and Metal-Y which has a specific heat capacity of 0.18 J/g °C. Now we take identical masses of each metal at the same temperature, and transfer the exact same amount of heat into each one. Which metal will reach the highest temperature?
- Metal-X
- Metal-Y
- they will have the same high temperatures
Thu 10/27
Isobutane (C4H10, MWt = 58 g/mol) has a heat of combustion of 2651 kJ/mol. How much heat is released when 7.25 grams of isobutane is burned?
- 534 kJ
- 662 kJ
- 295 kJ
- 482 kJ
- 920 kJ
- 331 kJ
- 176 kJ
- 727 kJ
- 846 kJ
Tue 10/25
Why would they (oil industry) actually burn off excess natural gas which directly makes CO2 to go into our atmosphere?
- because is is the cheapest thing to do
- because they are just evil
- because CO2 vs CH4 is a less impactful greenhouse gas
- because the burn-offs look pretty
- because they are making electricity out of the methane
Thu 10/20
Why don't nitrogen and oxygen act as greenhouse gases?
- because they don't absorb IR radiation
- because they don't absorb UV radiation
- because they readily decompose in visible light
- because they are toxic in the atmosphere
Tue 10/18
What is the molecular geometry of iodine tribromide?
- trigonal bipyramid
- T-shaped
- trigonal planar
- trigonal pyramid
- see-saw
Thu 10/13
Here is pyrethrin, an insecticide you spray to kill various pests (especially mosquitoes).
What is the best estimate of the bond angle that is indicated?
- 109.5°
- 90°
- 107°
- 118°
- 120°
- 180°
Tue 10/11
What's the formal charge of the marked atom?
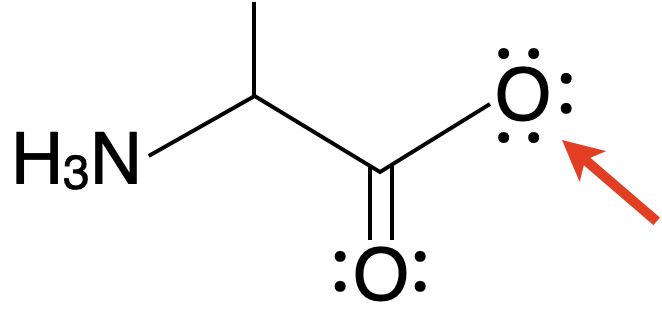
- +2
- +1
- 0
- –1
- –2
Thu 10/06
How many total valence electrons will be in the electron dot formula for phosphate ion (PO43–)
- 34
- 26
- 32
- 29
- 28
Tue 10/04
Phosphorus will commonly form 3 single covalent bonds. 5 such compounds are listed below. Which one will have the most polar bonds?
- PI3
- PBr3
- PH3
- PCl3
- PF3
Thu 9/29
Which ionic compound has the greatest attraction between the ions (this is effectively "lattice energy")?
Tue 9/27
What is the electron configuration of selenide ion (Se–2)?
- [Ar] 4s2 3d10 4p4
- [Kr] 5s2 4d10 5p4
- [Ar] 4s2 3d10 4p6
- [Ar] 4s2 3d8 4p6
- [Ar] 4s2 4d10 4p4
Thu 9/22
One of the following sets of quantum numbers (that define a specific atomic orbital) is not a valid set. Which one is it?
- \(n=4 ~~~~ \ell=2 ~~~~ m_{\ell}=-3\)
- \(n=3 ~~~~ \ell=2 ~~~~ m_{\ell}=-1\)
- \(n=2 ~~~~ \ell=1 ~~~~ m_{\ell}=0\)
- \(n=5 ~~~~ \ell=3 ~~~~ m_{\ell}=+2\)
- \(n=6 ~~~~ \ell=4 ~~~~ m_{\ell}=+3\)
Tue 9/20
Imagine two charged particles, one is a cation (positive charge, +), and one is an anion (negative charge, –). As they drift towards each other, coulombs law sets in. Which of the following is true about the overall energy of the two particles as they get closer and closer to each other?
- the energy is gradually increasing
- the energy stays about the same
- the energy is gradually decreasing
Tue 9/20
Imagine two charged particles, one is a cation (positive charge, +), and one is an anion (negative charge, –). As they drift towards each other, coulombs law sets in. Which of the following is true about the overall energy of the two particles as they get closer and closer to each other?
- the energy is gradually increasing
- the energy stays about the same
- the energy is gradually decreasing
Thu 9/15
Newer wifi routers transmit in the 5GHz range. What is the wavelength of the electromagnetic radiation used by a wifi router on a channel frequency of 5.47 GHz?
- 18.1 m
- 54.8 cm
- 548 μm
- 5.48 cm
- 1.81 m
Tue 9/13
Gas AB2C decomposes...
AB2C(g) → A(g) + 2B(g) + C(g)
If AB2C is originally at 12 bar and then fully reacts, what is the partial pressure of gas B after the decomposition? Assume volume and temperature stay constant
- 24 bar
- 32 bar
- 12 bar
- 16 bar
- 48 bar
Thu 9/08
Three gases (He, O2, and Ar) are in a container as shown in the diagram below. Each "dot" represents 1 mole of that gas. The 4, 32, and 40 are the molar masses of each gas.
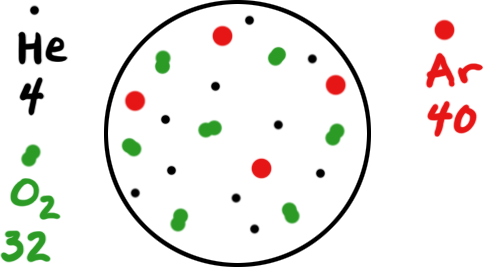
What is the mole fraction of oxygen in this mixture?
- 0.25
- 0.33
- 0.50
- 0.19
- 0.42
Tue 9/06
Which of the following gases is not a "pollutant" in our atmosphere?
- NOx
- O3
- CO2
- SOx
- VOCs
Thu 9/01
How many moles of gas are in a 10 liter container if the pressure is at 2 atm and the temperature is 30 °C?
- well below 0.2 mol
- 0.525 mol
- over 3 mol
- 1.32 mol
- 0.804 mol
Thu 9/01
placeholder dummy question - dr mccord is experimenting
- choice1
- choice2
- choice3
- choice4
- choice5
Tue 8/30
How many grams of CO2 are made when 79 g of C10H22 is burned?
- 282 g
- 245 g
- 310 g
- 222 g
- 210 g
Thu 8/25
Which one is a homogeneous mixture?
- concrete in sidewalk
- milk
- Kool-Aid (glass of cherry / no ice)
- choc. chip cookie
- water with sand in it
Tue 8/23
FIRE is HOT!
- true
- false