Exam 2
Tuesday 10/4
7pm - 8:30pm
WEL 3.502
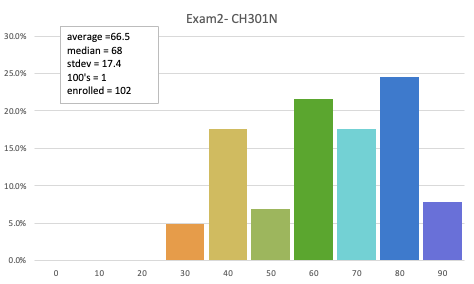
66.5 avg (raw)
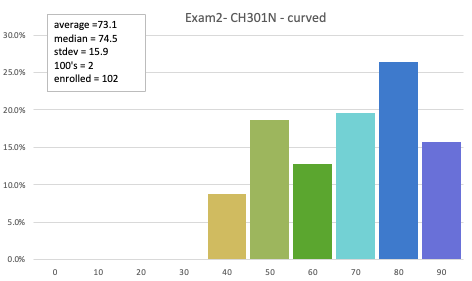
73.1 avg (curved)
🔑 Here are all the KEYS to Exam 2
Learning Outcomes
Students will know...
- what electromagnetic radiation is and how we chunk it up into seven regions
- the order of the regions of EM radiation from highest to lowest
- the order of the colors of the visible spectrum and the two wavelengths we use to define it
- how matter interacts with each region of the EM spectrum
- the names and rules for the four quantum numbers that describe electrons within atoms
- how to write the electron configuration for any element on the periodic table
- how to write the electron configuration for any monatomic ion
- how to correctly name, and write the full formula for polyatomic ions ("shortlist" in section 3.08)
- how to name cations and anions (including polyatomic ions) and ionic compounds (salts)
- the general trends we covered on the periodic table: atomic radii, monatomic ion radii, ionization energy, electron affinity, and electronegativity
- how to identify and name simple covalent compounds
- how to distinguish between ionic bonds, perfectly covalent bonds, and polar covalent bonds
- what the determining factor is to have a polar molecule
- anything I covered, talked about in class, that I forgot to list here
Formulas YOU should know
wavelength and frequency
\(c = \lambda \cdot \nu \)
Planck's Equation / Photon energy
\(E = h\nu \)
Jimmy's Help Videos on Exam 2 topics
Exam protocol All you need to know about HOW to take our in-class exams.