McClicker Questions for the Semester - if you don't know the answer, go to the class notes for that day and find the McClicker question.
Order is now in REVERSE. Most recent at the top.
Mon 11/28
Which one of the following is the best indicator of a reaction being at equilibrium?
- ∆Srxn > 0
- the reaction is exothermic
- moles of reactants equals moles of products
- ∆Grxn = 0
- the forward and reverve reactions stop
Fri 11/18
We often refer to "one mole of standard reaction" when calculating thermodynamic changes in state. Which statement is true about any "standard reaction"?
- A standard reaction can only go in the forward direction.
- A standard reaction can go in either direction, forward or reverse.
- A standard reaction can only go in the reverse direction.
Wed 11/16
Consider a chemical reaction that has reached equilibrium. The reaction is endothermic and has a positive entropy change. Which choice best represents the response to temperature change for this reaction?
- The described condition is impossible - you can't have an endothermic reaction with a positive entropy change be at equilibrium.
- As the temperature rises, the reaction makes more reactants.
- There can be no temperature dependence because it is already at equilibrium.
- There is no way to predict any change in the reaction with a temperature change.
- As the temperature rises, the reaction makes more products.
Mon 11/14
Consider the physical change of freezing liquid water into ice at a temperature of 4 °C. Consider the overall process from an entropy standpoint. Now, which value has the greatest magnitude, ΔSsys or ΔSsurr? To be clear, you are comparing the absolute value of these two changes in entropy.
- ΔSsys is the greatest
- they are identical in magnitude
- ΔSsurr is the greatest
Fri 11/11
Calculate the standard entropy of formation of methane using thermodynamic table data.
- 🐶 –76.0 J/mol K
- 🦊 +186 J/mol K
- 🐼 +49.26 J/mol K
- 🦀 –81.74 J/mol K
- 🐞 –91.34 J/mol K
Thermo Data Here is a link to the gchem thermo tables.
| substance | ΔHf° | ΔGf° | S° |
|---|---|---|---|
| C(s) (graphite) | 0 | 0 | 5.740 |
| C(s) (diamond) | 2 | 3 | 2 |
| CH4(g) | −75 | −51 | 186 |
| H2(g) | 0 | 0 | 131 |
| H(g) | 217 | 203 | 115 |
| H+(aq) | 0 | 0 | 0 |
Wed 11/09
What is \(\Delta S\) for the heating of 2.5 moles of ideal gas from 300 K to 390 K?
- +8.2 J/K
- +3.3 J/K
- –8.2 J/K
- –3.3 J/K
- +2813 J/K
Mon 11/07
Estimate the \(\Delta H_{\rm rxn}\) for the following reaction using bond energies.
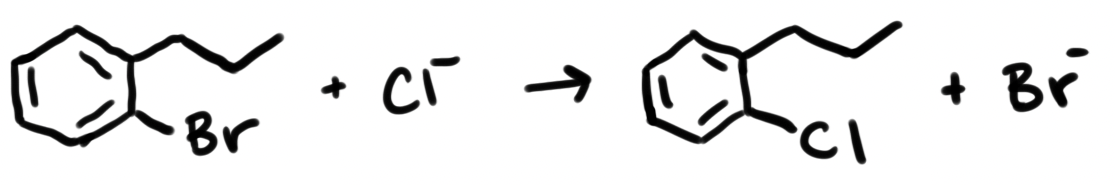
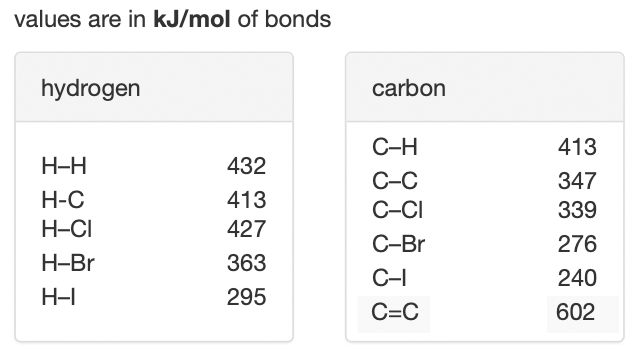
- +615 kJ
- -615 kJ
- -63 kJ
- +63 kJ
- -157 kJ
- +54.08 kJ
- –54.08 kJ
- –5.72 kJ
- +5.72 kJ
- +17.81 kJ
- –17.81 kJ
- Metal-X
- Metal-Y
- they will have the same high temperatures
- 31.4 kJ
- 170 kJ
- 25.1 kJ
- 18.7 kJ
- 72.1 kJ
- 534 kJ
- 662 kJ
- 895 kJ
- 482 kJ
- 920 kJ
- 188 kJ
- 331 kJ
- 727 kJ
- 246 kJ
- +200 kJ
- –200 kJ
- +300 kJ
- –300 kJ
- +250 kJ
- –250 kJ
- VP will be higher
- VP will stay the same
- VP will be lower
- it's a trick! pick me!
- X , Tbp = 69 °C
- Y , Tbp = 84 °C
- Z , Tbp = 54 °C
- they all have the same VP at room temperature
- CBr4
- CI4
- CF4
- CCl4
- they are all the same
- σ2s*
- π2p
- σ2p
- π2p*
- σ2s
- σ2p*
- sp3 109.5°
- sp3d 120°
- sp2 112°
- sp3 107°
- sp2 120°
- sp 180°
- see-saw
- trigonal bipyrimid
- tetrahedral
- square planar
- cubic
- –2
- –1
- 0
- +1
- +2
- 8
- 6
- 4
- 7
- 10
- 34
- 32
- 26
- 29
- 28
- PI3
- PBr3
- PH3
- PCl3
- PF3
-
↿⇂ ↿
-
↿ ⇂ ↿
-
↿ ↿ ↿
-
↿⇂ ↿⇂ ↿⇂
- they are all the same
- 0
- 1
- 2
- 3
- 4
- \(n=4 ~~~~ \ell=2 ~~~~ m_{\ell}=-3\)
- \(n=3 ~~~~ \ell=2 ~~~~ m_{\ell}=-1\)
- \(n=2 ~~~~ \ell=1 ~~~~ m_{\ell}=0\)
- \(n=5 ~~~~ \ell=3 ~~~~ m_{\ell}=+2\)
- \(n=6 ~~~~ \ell=4 ~~~~ m_{\ell}=+3\)
- 18
- 8
- 36
- 16
- 32
- 50
- 560 nm
- 488 nm
- 244 nm
- 675 nm
- 216 nm
- 18.1 m
- 54.8 cm
- 548 μm
- 5.48 cm
- 1.81 m
- 40 bar
- 26.6 bar
- 10 bar
- 13.3 bar
- 20 bar
- 600 atm
- 500 atm
- 300 atm
- 200 atm
- 750 atm
- gas A
- gas B
- gas C
- they all have the same K.E.
- can't answer - must know molar masses
- 8 bar
- 12 bar
- 16 bar
- 6 bar
- 9 bar
- 16 atm
- 80 atm
- 40 atm
- 36 atm
- 60 atm
- X is limiting, with 2.7 g of Y leftover
- Y is limiting, with 2.1 g of X leftover
- X is limiting, with 1.6 g of Y leftover
- Y is limiting, with 8.2 g of X leftover
- X is limiting, with 5.2 g of Y leftover
- true
- false
Fri 11/04
The destruction of the ozone layer in our atmosphere can be partly shown by the combination of the following reactions.
ClO + O3 → Cl + 2O2 –29.90 kJ
2O3 → 3O2 +24.18 kJ
What is the ΔH for the following net reaction?
Cl + O3 → ClO + O2 ? kJ
Wed 11/02
I have two metals to compare. Metal-X which has a specific heat capacity of 0.36 J/g °C, and Metal-Y which has a specific heat capacity of 0.18 J/g °C. Now we take identical masses of each metal at the same temperature, and transfer the exact same amount of heat into each one. Which metal will reach the highest temperature?
Mon 10/31
How much heat is needed to melt 75.0 g of ice at 0 °C?
Fri 10/28
Isobutane has a heat of combustion of 2651 kJ/mol. How much heat is released when 7.25 grams of isobutane is burned?
Wed 10/26
A chemical reaction run and found to release 250 kJ of heat while also doing 50 kJ of work. What is the value for ΔU for this system?
Mon 10/24
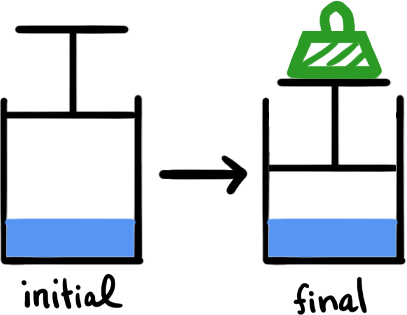
A piston and cylinder system is initially set up such that it is one quarter full of liquid A and the rest is the space above the liquid with the vapor pressure of A in it. Then the piston is pushed down with a weight which reduces the volume above the liquid (head space) to about half of its previous value. This is the final condition. Which statement best describes the vapor pressure (VP) of liquid A in the final state compared to its vapor pressure in the initial state?
Fri 10/21
Consider the 3 liquids X, Y, and Z. Below they are listed with their normal boiling points. Which liquid of these three will have the highest vapor pressure at room temperature?
Wed 10/19
Consider the carbon tetrahalides below. Using your knowlege of IMFs, which one will have the highest boiling point?
Mon 10/17
Which of the following molecular orbitals (MOs) is the HOMO for the diatomic molecule B2?
Fri 10/14
Here is pyrethrin, an insecticide you spray to kill various pests (especially mosquitoes).
What is the hybridization and the bond angle of the indicated atom () in the structure?
Wed 10/12
Write out the electron line/dot formula for sulfur tetrafluoride and correctly identify the molecular geometry.
Mon 10/10
What's the formal charge of the marked atom?
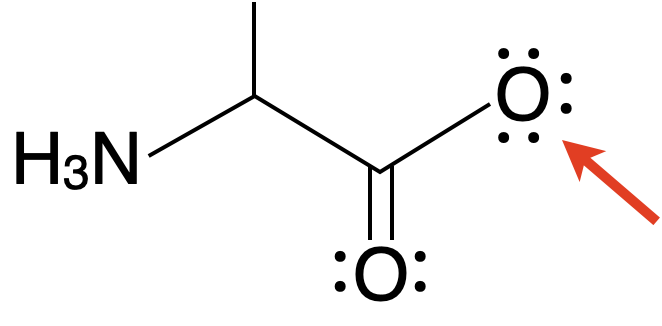
Fri 10/07
Consider the line structure shown below. How many H atoms are in this structure?
Wed 10/05
How many total valence electrons will be in the electron dot formula for phosphate ion (PO43–)
Mon 10/03
Phosphorus will commonly form 3 single covalent bonds. 5 such compounds are listed below. Which one will have the most polar bonds?
Fri 9/30
Which filling diagram is the correct one for the outermost p orbital set that has electrons for antimony (Sb)?
Wed 9/28
Which ionic compound has the greatest attraction (lattice energy)?
Mon 9/26
How many angular nodes are in a 5d orbital?
Fri 9/23
One of the following sets of quantum numbers (that define a specific atomic orbital) is not a valid set. Which one is it?
Wed 9/21
What is the maximum number of electrons possible in an atom with n = 4 ?
Fri 9/16
A photon with enough energy, 5.1 electron volts (eV) of energy to be precise, will eject an electron from a piece of gold. What frequency and wavelength does light with this energy have? Note: 1 eV = 1.60 × 10-19 J.
Wed 9/14
Newer wifi routers transmit in the 5GHz range. What is the wavelength of the electromagnetic radiation used by a wifi router on a channel frequency of 5.47 GHz?
Mon 9/12
Gas AB2C decomposes...
AB2C(g) → A(g) + 2B(g) + C(g)
If AB2C is originally at 10 bar and then fully reacts, what is the partial pressure of gas B after the decomposition? Assume volume and temperature stay constant
Fri 9/09
Attractive forces reach farther than repulsive forces. But as molecules get closer and closer, the repulsive forces will dominate. Look at the figure for the compression factor, Z. At approximately what pressure do the repulsive forces of CO2 EQUAL the attractive forces?
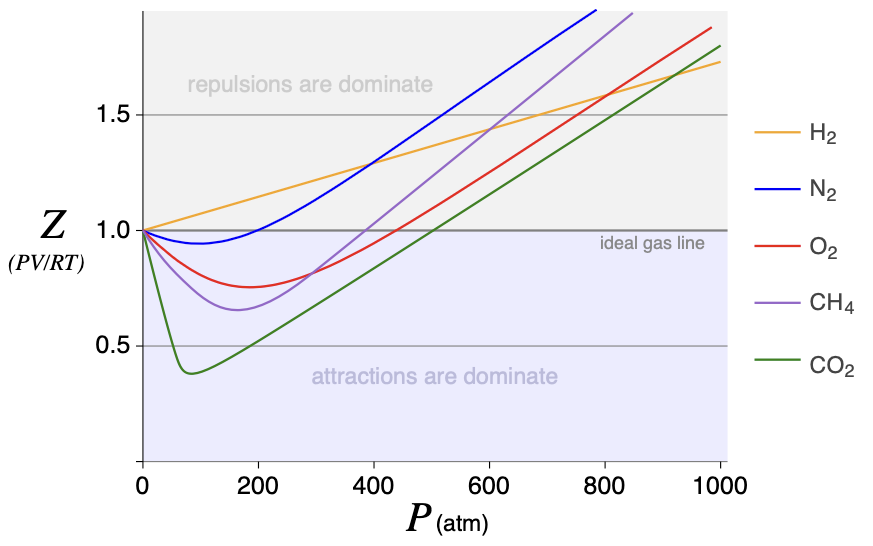
Wed 9/07
Three identical containers (same volume and temperature). Each has a gas in it as shown (modeled) by the dots in each. The size of the dots also models the relative sizes of the gas molecules. Each dot represents the same number of moles in each container as well.
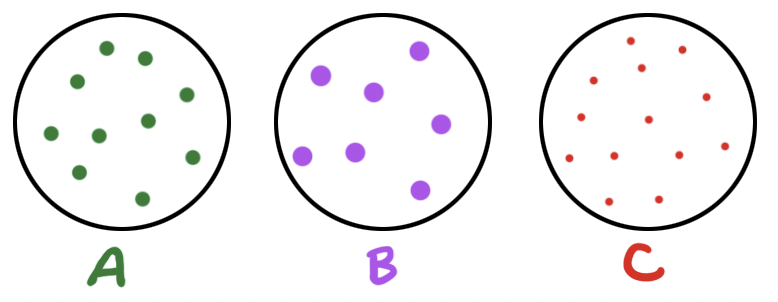
Which container/gas has the greatest kinetic energy (J/mol) for the contained gas?
Fri 9/02
5 moles of hydrogen and 15 moles of nitrogren are put into a container such that the total pressure is 36 bar. What is the partial pressure of hydrogen in this mixuture?
Wed 8/31
If 50 L of an ideal gas at 8 atm is compressed to 10 L, what is the new pressure of the gas?
Fri 8/26
Consider the reaction:
2X + 5Y → 4Z
20 g of X (60 g/mol) is allowed to completely react with 36 g of Y (40 g/mol) and make Z (80 g/mol). Which of the two reactants is limiting and how much of the other reactant is leftover?
Wed 8/24
FIRE is HOT!