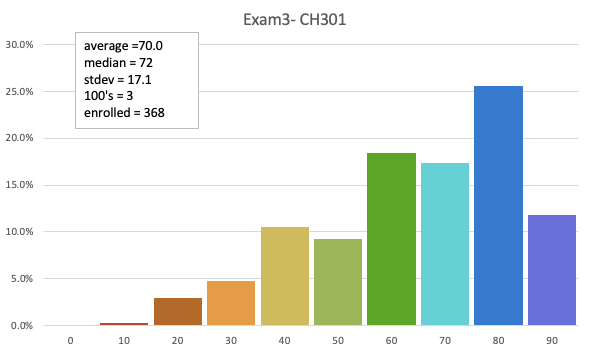
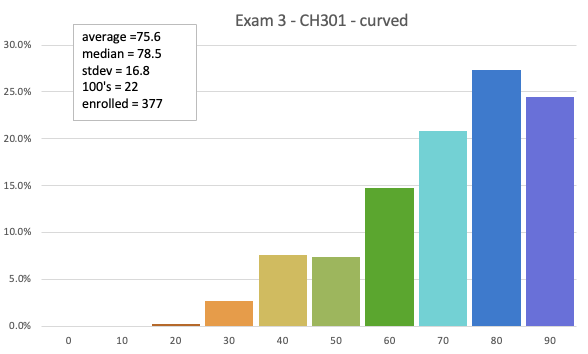
Exam 3
Tuesday 10/25
7:00pm - 8:30pm
70.0 avg (raw)
75.6 avg (curved)
🔑 Here are all the KEYS to Exam 3
Note: these are from Exam 2 - but you still need to know them
You should also know nomenclature but it will not be specifically tested.
Students will be able to...
- Identify metals and non-metals, and predict the types of compounds (ionic/covalent) that will form from different elements.
- Distinguish between molecules, ions, and atoms.
- Predict the anion or cation that a main-group element is likely to form.
- Relate Coulomb’s law to ionic radii, ionic charge, and lattice energy.
- Nomenclature IONIC compounds: YES, you will be asked to name an ionic compound and also be given a name and you pick the correct formula. Yes, ALL the polyatomic ions are fair game here.
- Nomenclature COVALENT compounds: Name and write formulas for simple covalent compounds containing 2 different non-metals (like CO is carbon monoxide).
- Rank the polarity of covalent bonds based on relative electronegativity. This is knowing the relative values of ΔEN based on positions in the periodic table.
Chapter 4 - Bonding (Exam 3 material / Sections 4.3 - 4.10)
NOTE: Here are the rest of the outcomes from chapter 4. You will be tested on these outcomes.
- Describe the distance dependence of the potential energy of a covalent bond.
- Predict and explain relative bond strength and lengths in a compound.
- Interpret line drawings of chemical compounds with implicit hydrogens, carbons, and lone pairs.
- Draw the line structure for simple organic compounds with just C, H, O, and N in it. Ok, maybe a halogen or two as well (F, Cl, Br, I).
- Define dipole moment and identify polar bonds.
- Draw the best Lewis structure (including any resonance structures) for a molecule or polyatomic ion.
- Apply formal charges to structures and use them to predict the most likely structure.
- Recognize and apply exceptions to the octet rule.
- Apply the VSEPR model to determine a molecule's electronic and molecular geometry based on its Lewis dot structure.
- Assess if a molecule is polar based on polar bonds and its molecular geometry.
- Identify the orbital hybridization for any atom in a given molecule using the VB model.
- Describe the type of bond (e.g. sigma, pi) and the atomic orbitals that are associated with the bond using the VB model.
- Differentiate between localized and delocalized electrons within a structure.
- Diagram orbital hybridization using orbital notation.
- Recognize that Molecular Orbital (MO) theory is used to determine the energy of the electron in a molecule as well as its geometry.
- Differentiate between constructive interference and destructive interference of atomic orbitals.
- Construct and fully interpret a MO diagram, including identifying the bond order, the lowest energy electronic excitation energy (HOMO-LUMO gap), and the magnetism (paramagnetic or diamagnetic) for a compound.
- Construct and use a MO diagram for all diatomic molecules and ions (X2, X2+, X2–, etc...) based on the first 10 elements of the periodic table.
- Recognize and use the proper notation for electron configuration of molecular orbitals. Example: \( \sigma_{\rm 1s}^2 \; \sigma_{\rm 1s}^{*2} \; \sigma_{\rm 2s}^2 \; \sigma_{\rm 2s}^{*2} \; \pi_{\rm 2p}^2 \)
Learning Outcomes for IMFs
Students will be able to...
- Define the three major intermolecular forces (IMFs) that can exist in condensed phases: dipole-dipole, hydrogen bonding, and dispersion.
- Predict the types of IMFs that a compound can exhibit based on its structure.
- Using bonding theories and IMFs, predict the chemical and physical properties of organic materials.
- Explain how size, shape and polarizability affect the magnitude of dispersion forces.
- Relate the IMFs of a compound to liquid properties such as boiling point, vapor pressure, viscosity, and surface tension.
- Explain how liquid properties vary with temperature.
- Fully describe (atomic arrangement/microscopic view) and visually depict the four types of solids (covalent, ionic, metallic, molecular).
- Summarize how the macroscopic properties of solids (e.g. melting point, hardness, conductivity) can be explained by the microscopic model of solids.
- Use physical data to deduce the type of bonding within solids.
VSEPR Theory
Empirical. Shapes are predicted via "common sense" about electron regions repulsing each other.
Dr. McCord's VSEPR Help Site - a mini review site with all things VSEPR and a little VB to boot.
VB Theory
The geometries needed for compounds are made by combining atomic orbitals into hybrid orbitals with the corresponding geometries. Sigma and pi bonding are introduced and are a key component of this bonding theory. All hybridizations are localized on the central atoms.
MO Theory
All of the atomic orbitals for the entire set of atoms in the molecule are used to create a new set of molecular orbitals. MO theory is much more "whole-istic" meaning it includes all the nuclei and electrons to make molecular orbitals. The sigma and pi bonding concept is still in place, but antibonding orbitals are introduced as well.
Intermolecular Forces (IMFs)
Intermolecular forces are the forces that are between molecules. They are the forces that hold liquids and solids together - collectively known as cohesive forces.
dipole-dipole
All polar molecules have dipole-dipole forces of attraction. All the partial positive and negative charges pull the molecules together.
H bonding
A special case version of dipole-dipole that is much stronger than "plain" dipole-dipole. A partially positive H must be covalently bonded to a nitrogen, oxygen, or fluorine atom in order to have H-bonding.
dispersion forces
All molecules have dispersion forces. For non-polar molecules, dispersion forces are the only IMFs present. Dispersion forces are the weakest of the three forces when compared one to one in small molecules. However, dispersion forces scale with molecular size (surface area actually). So all large molecules (and atoms) tend to have large dispersion forces - so much so that all very large molecules are solids.
Exam protocol All you need to know about HOW to take our in-class exams.