exam 1
9/18
exam 1
9/18
Wednesday 9/18
2-2:50pm
UTC 2.102A
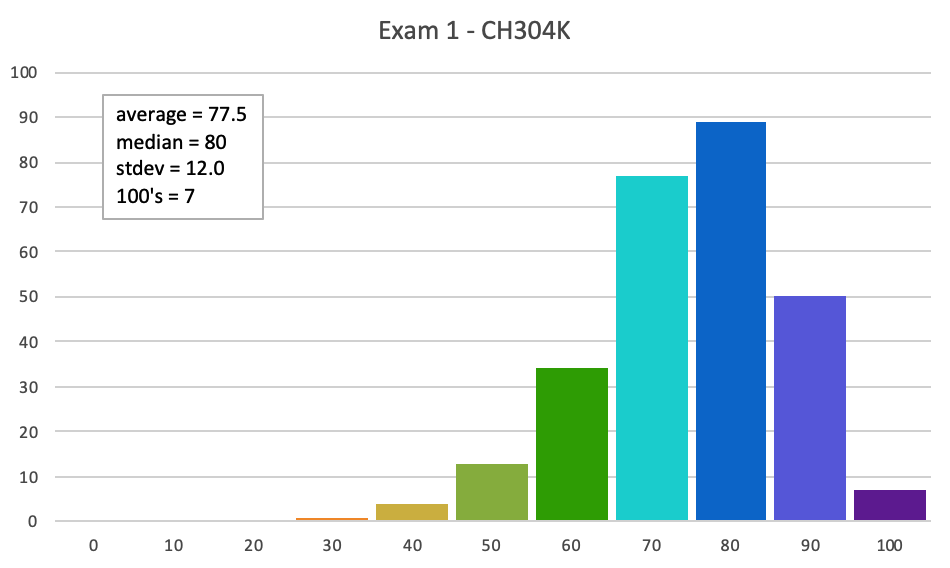
What we provide on Exams We will provide all students with:
The cover page with the periodic table will be very similar to the one available in the Appendix of the ChemBook website (Chapter 10).
Coverage: Exam 1 covers all the material that was covered on HW's 01 and 02. ChemBook Chapters: The exam covers all of Chapters 1 and 2 which is some fundamentals of chemistry plus gas laws and some atmosphere/air knowledge.
Questions: The exam will have 20 multiple choice questions. This means that each question is worth 5 points. We might decide to push that ±1 point for a few questions - meaning 4 and 6 points for a handful of questions. The point values are included with all questions so you'll see the points. We will only grade you by what is bubbled in on the answer sheet that you turn in. We will not look at your exam copy for answers, nor consider them in any way. Bubble carefully and correctly.
Students will know...