

Average 49970: 74.55
Avegage 49975: 74.29
ALL Average: 74.43
Median: 76
Std Dev: 17.36
Total 100's: 23
bubblesheet errors: 11
For a nicer more annotated version of the formulas, click on smiley there 🙂
ΔU = Uf - Ui
ΔU = q + w
q = m Cs ΔT
q = n Cm ΔT
q = m ΔHtrans
q = n ΔHtrans
w = -PextΔV
w = -ΔngasRT
Δngas = (#mol gas prod) - (#mol gas react)
H = U + PV
ΔH = ΔU + PΔV
ΔU = ΔH - PΔV
ΔU = ΔH - ΔnRT
ΔH = qP
ΔU = qV
q = nRT ln(V2/V1)
w = –nRT ln(V2/V1)
qcal = -qsys
qcal = CcalΔT
qcal = mwater · Cs,water · ΔT + Chardware · ΔT
ΔHrxn = ΔH1 + ΔH2 + ΔH3 + ...
ΔHrxn° = ΣnΔHf° (products) - ΣnΔHf° (reactants)
ΔHrxn° ≈ ΣnΔHbond°(breaking) - ΣnΔHbond°(making)
ΔSuniv = ΔSsys + ΔSsurr
S = k ln Ω
ΔS = qrev / T
ΔS = n Cp ln(Tf / Ti)
ΔS = nR ln(V2/V1)
ΔStrans = ΔHtrans / Ttrans
ΔSrxn° = ΣnS° (products) - ΣnS° (reactants)
G = H - TS
ΔG = ΔH - TΔS
ΔGrxn° = ΣnΔGf° (products) - ΣnΔGf° (reactants)
ΔH = TeqΔS
We will provide all data for the exam. Specifically, we will provide a table of thermodynamic values, a table of phase change data, and a table of bond energies. We will also provide values for any constants and conversions needed.
Here is what the bond energy tables look like...
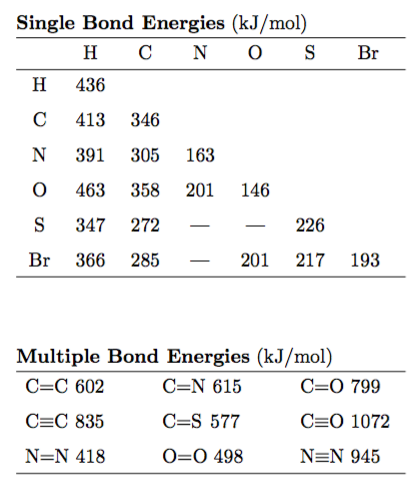
Here is what the thermo tables will look like - although different substances will be provided...
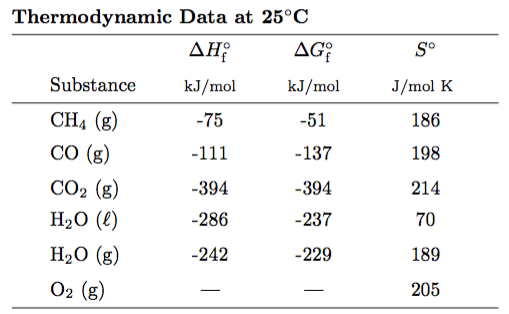
We might even add molar heat capacities (CP,m) to this table as well. And by-the-way... CP,m is the molar heat capacity at constant pressure.
We will provide the data in some form of table. Be prepared to use any such table of data.
And now, once again, for your enjoyment...
Students will be able to...