Schedule1 Schedule2 Schedule3 Schedule4
Schedule to Exam 1
| Date | Day | Topics |
|---|---|---|
| 8/27 | Thu | 1st class day. Go over syllabus. Talk about ALEKS. Talk about iclickers. Class assignments. etc. Maybe introduce Chapter 12 on Atomic Theory. |
| 8/28 | Fri |
| 8/31 | Mon | |
| 9/1 | Tue | Officially start Chapter 12 in the book - Atomic Theory. Electromagnetic radiation. Light as a particle and a wave. Planck's Constant. EM radiation and matter. DeBroglie equation. |
| 9/2 | Wed | ALEKS Assessment DEADLINE!! (by 5PM) |
| 9/3 | Thu |
Deadline to finish ALEKS assessment or be dropped from course (by 5PM)
Discussed emission spectra and absorption spectra for hydrogen. Also known as line spectra which led to the realization that energy is quantized within the atom. 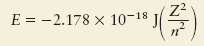 Found
energy levels for hydrogen and numbered them 1, 2, 3, 4,... These levels are calculated via equation
12.1 in your book (show to the right here). Found
energy levels for hydrogen and numbered them 1, 2, 3, 4,... These levels are calculated via equation
12.1 in your book (show to the right here). 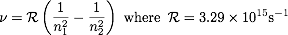 Defined the Balmer Series and the Lymann
Series for hydrogen spectra. Rydberg constant and formula allows one to predict the differences in
the levels. To the right is the Rydberg equation where it calculates for the frequency of the
energy difference in two levels n1 and n2. n1 and n2 are 1, 2, 3, 4, 5, 6, etc... n2 is an UPPER
level while n1 is a lower level. Multiply that frequency by Planck's constant (h) and you've
got E.
Defined the Balmer Series and the Lymann
Series for hydrogen spectra. Rydberg constant and formula allows one to predict the differences in
the levels. To the right is the Rydberg equation where it calculates for the frequency of the
energy difference in two levels n1 and n2. n1 and n2 are 1, 2, 3, 4, 5, 6, etc... n2 is an UPPER
level while n1 is a lower level. Multiply that frequency by Planck's constant (h) and you've
got E.
Started discussion about Schrodinger equation. Looked at the parts and discussed them. Then said we would start "easy" by solving the equation for what is know as the "Particle in a Box" or PIAB. Will have to continue PIAB on Tuesday, 9/8. |
| 9/4 | Fri |
| 9/7 | Mon | Labor Day - Holiday - no class |
| 9/8 | Tue | Finish PIAB. Relate this to electrons in a hydrogen atom. Wavefunctions and Quantum numbers |
| 9/9 | Wed | Homework 2 is due by 11:30 PM |
| 9/10 | Thu | Splitting wavefunctions into parts. Shapes of orbitals. Electron Configurations. |
| 9/11 | Fri | Homework 1 is due by 11:30 PM |
| 9/14 | Mon | |
| 9/15 | Tue | Electron Configurations. Talk about Exam 1. Isoelectronic species -
their e- configurations (all the same) and their sizes. Periodic Table trends: ionization energies,
electron affinities, atomic radii. Also discussed iclicker registrations.
Homework 3 is due by 11:30 PM |
| 9/16 | Wed | EXAM 1 : 7-9 PM |