Preparing for Exam 3
Exam 3 Question Types
Suprise, there are 25 questions and they will be the following...
- The chemical system and state functions
- Famous gas laws
- Partial pressure (Dalton's Law) calculation
- Combined gas law calculation
- Using PV = nRT to calculate MW
- Relating number density to pressure
- Gas reaction stoichiometry - combining volumes
- Gas reaction stoichiometry - partial pressures
- Kinetic Molecular Theory
- Maxwell distributions - graph interpretation
- Graham's Law of Effusion - ratio of gas speeds vs masses
- Gas non-ideality - theory - failing conditions of the IGL
- Gas non-ideality – van der Waals coefficients - meaning and use
- Distinguishing intra and Intermolecular forces
- Intermolecular force theory (dispersive forces)
- Intermolecular force theory (H bonding)
- Assigning Intermolecular force in molecules
- Assigning Intermolecular force in molecules
- Theory behind ranking of properties of liquids (dispersive forces)
- Theory behind ranking of properties of liquids (H bonding)
- Physical property definitions
- Ranking properties of liquids (direct relationship with IMF)
- Ranking properties of liquids (inverse relationship with IMF)
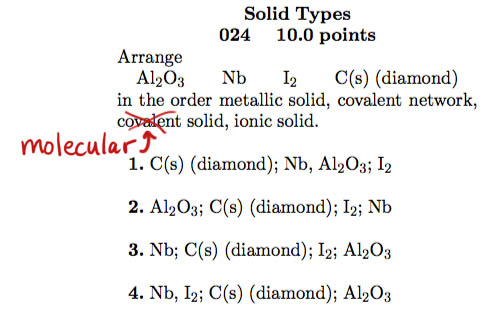
- Types of solids
- Ranking properties of solids (melting point)
Practice Exam 3 and Key
Important: Remember to try and learn ALL the material for the exam first. Then, set aside a 2 hour time slot and take the practice exam. Do not use any extra resources, just you, your brain, a calculator, and a pencil.
Note: The cover page with formulas has been separated from the practice exam.
Error on #24 of practice exam. The term "covalent solid" in the question is supposed to be "molecular solid".