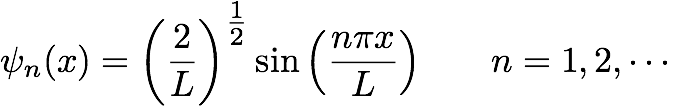Review Sheet - Exam 1 - CH301 - McCord
Which Chapter/Sections are covered?
Chapters 1-4, the “Fundamentals” were covered on homeworks 1 and 2 (not to mention, ALEKS). I DO plan on asking at least ONE question from the fundamentals (stoichiometry most likely), however, the exam is based on content in Chapter 12. All the material on the exam has been covered in one way or another on homeworks 3 and 4 and my lectures. Concentrate on the subject matter emphasized in class and on these homeworks. If I didn't cover it in class, it will NOT be on the exam.
Come in mentally prepared to answer at least 25 questions, maybe a few more. Yes, there will be calculations, but most of the questions will be over theory and concepts. You need to understand the theory and concepts to the level at which we studied it. I don’t expect you to solve the Schrödinger equation, but I do think you should understand what it meant towards modern atomic theory. And, just because you got a homework question right does not necessarily mean you really understand the material. Try explaining the concepts to someone else to see if you really understand.
- Bring a pencil(s) and non-graphing calculator to the exam. WE will provide you with an exam copy, an answer sheet (bubblesheet), and any scratch paper that you might need.
- I was going to have you memorize formulas, like 3 or 4 of them, but then I decided to just print them on the exam anyway. Formulas will be on the exam as well as ALL constant needed in the formulas. There might even be some extra constants and formulas, so just use the necessary formulas and constants.
Energy traveling at the speed of light
Know what electromagnetic radiation is and how we depict it on the page and conceptually.
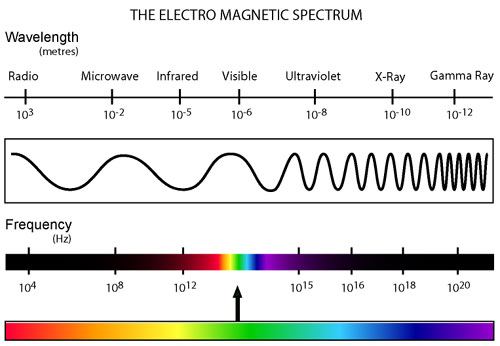
Know the basics of the entire electromagnetic spectrum (Figure 12.3 in your book is much like the one shown here which I grabbed off the internet).
Know the approximate wavelengths for each type of radiation given (LOOK at that figure). Know also, that visible light is in the 400-700 nm range (that’s blue end to red end). Other than the two ends, I do not expect you to know the wavelengths of all the colors of the rainbow – however, you SHOULD know the ordering of the colors (think Roy G. Biv).
Use Your Resources - the Internet
Type in “electromagnetic radiation” into a google image search. LOTS of good pictures for learning the relative areas of the scale. Do know what type of excitations go with the various radiation types. For example, visible and UV light yield electronic excitations in atoms and molecules while infra-red (IR) radiation really just causes vibrational excitations. The internet is your friend - use it and you can find countless examples of ALL of this material.
EM Radiation Equations
Know the two views of electromagnetic radiation: as a wave and as a particle (photons). This is best summed up by the two basic equations that we have used to describe electromagnetic radiation:
c = λ ν E = h ν
Know how to use this equations to calculate various wavelengths, frequencies, and energies of photons. And yes, both c and h values are given on the back of your bubblesheet.
Do all moving particles have wavelength?
Yes. Louis de Broglie said if light can be treated as a particle (photon) then why not the other way around? Any moving particle with mass and velocity should have a corresponding wavelength.
λ = h/p = h/mv
The 'p' given there is momentum of the particle which is equivalent to mass times velocity (mv). The most important moving particle for us is the electron (m = 9.11 × 10-31 kg). Now we can treat the electron as a wave. When confined to the region around the nucleus, the electron behaves as a standing wave.
What is the essence (observations) of the photoelectric effect?
How did Einstein explain this effect?
How does this relate to the work function (Φ) of a metal:
½ mv2 = hν - Φ
Be able to calculate any part of this equation (m, v, ν, or Φ) when given any 3 of the 4 variables in it. Realize that in our textbook (section 12.2), Zumdahl shows the work function term, Φ, as hν0. Also be careful here with 'nu' (ν - frequency) and 'velocity' (v). The symbols look a lot alike but are very different quantities. We will spell out "velocity" or "frequency" on an exam and there will be no ambiguity.
The Emission Spectrum of Hydrogen
Check out those lines. Lines I tell you. Not broad bands of continuous wavelengths but very very narrow precise lines. It’s like hydrogen is speaking to us. What is it saying? It is saying, “I have quantized energy levels!” “When I’m excited, I spit out very specific quanta of energy.” Who listened? Several smart guys but lets chalk one up for Bohr for realizing that the photon that is emitted is due to the energy difference in quantum levels within the atom. As an electron falls from an excited state to a lower energy state, a photon is emitted that corresponds to the energy gap. Balmer looked at the visible spectrum of hydrogen and wrote out an equation to fit the emission lines. Shortly after that, Rydberg came up with a more general equation for the energy levels within the hydrogen atom:
En = −ℝ / n2
Where the Rydberg constant (ℝ) is equal to 2.178 × 10-18 J (this is what is in your book in section 12.3). You can also divide that by h and have ℝ expressed as a frequency instead of energy, that value is 3.29 × 1015 s-1. You can use this equation to calculate all kinds of energies associated with the electronic transitions between the various levels in the hydrogen atom.
Let’s now count how many different atoms it works for… hmmmm It will work really well as long as you have a nucleus with a charge of +1 and ONE electron somewhere near it. OK, well that’s hydrogen and now your done - answer 1 atom type. OK, so we are somewhat limited here in our equation. The good that comes out of this is that we are now starting to get a more quantitative feel for how energy is in fact quantized within the atom. Note that the 'n' in that equation is essentially a quantum number. So how do we get a better equation for more electrons? Unfortunately, we will need to increase the complexity of the equation. So yes, the complexity will steeply rise as we proceed through the periodic table and investigate atoms with far more than one electron. The key here is that there WILL be an equation that WILL have quantum numbers associated with it. A better way to go about getting the right equation is to utilize the Schrödinger equation. OK, don’t just utilize it, SOLVE it and find the proper wavefunction (ψ) for the electron that you are interested in.
Schrödinger’s Equation
Impressive, isn’t it? Can you say differential equation? I knew you could. What’s it for? What comes out of it? Can it be solved exactly for all atoms?
The solving of this equation totally depends on setting it up properly. We must mathematically define the boundary conditions for this equation. We’ll use spherical coordinates for this (r, θ, φ) as described in section 12.7 in your book. Oh my! Check out some of the answers (&psi’s) that come out of this thing. (Table 12.1) Scary isn’t it. Maybe we should hang on a minute and handle a much easier system with only ONE dimension (not three) and no potential energy. Of course I’m referring to the particle-in-a-box (PIB) problem.
Particle in a Box - Standing Waves
Yes, if we confine a particle, in a very very small space, its wavelike behavior starts taking over. Only certain waves will fit into the “box”. Tada! These waves are quantized. Each wave has a specific wavelength and energy associated with it. Oh yeah, our “box” has only one dimension, length - not really a box at all is it? Some books call it a particle-on-a-line - better name? Not the historical name though. Anyway… You can solve this problem using the Schödinger equation. Since there is only 1 dimension in this problem (x), there is only one quantum number (n) to use in the wave equations, &psi. You get the following solution(s):
This is basically fitting various oscillations of a sine function into a finite length, L. These wavefunctions are clearly shown in your book and I showed them in class also. The key thing to notice is that you get nodal points along the wavefunction. The number of nodal points is always equal to n-1. Now, this wavefunction can then be solved for energy:
How would these solutions look graphically? See Figure 12.14 in section 12.6.
So why did go to the trouble of solving for PIB? What can be learned from this little exercise? The idea is that this a nice “friendly” segue into the various solutions to the Schrödinger equation. Compare and contrast the similarities of this equation and the equation that Schrödinger arrived at for the energy levels within the hydrogen atom.
Schrödinger for the atom required 3 dimensions. You will get a quantum number for each dimension you use in the Schrödinger equation. We use 3 dimensions so there are 3 quantum numbers: n, ℓ, and mℓ. These combined with the wavefunction, &psi, describe the SPACE that the electron is occupying for a given level of energy. Later, another property of the electron had to be accounted for which was spin and the 4th quantum number, ms was born.
Quantum Numbers
Know the names, symbols, and values (rules) for the four quantum numbers n, ℓ, and mℓ, ms. Also know what each one represents in terms of the electron orbitals of the atom.
- principle, n = 1, 2, 3, …
- angular momentum, ℓ = 0, 1, 2, … n−1
- magnetic, mℓ = −ℓ … −1, 0, 1, … ℓ
- spin, ms = +½, −½
Out from these quantum numbers and the solution to the Schrödinger equation comes wavefunctions. What does the wavefunction, ψ, tell us? Ultimately it allows us to map out in three dimensions the likelihood of finding an electron in a given amount of space. This is what gives us the orbitals of the hydrogen atom that we are (now) all familiar with.
Know your orbitals: name, quantum numbers, shape, numbers, nodal surfaces
Know the basic differences in each orbital type: s, p, d, and f. You should even be able to follow the trend to g, h, i, etc… What do nodes have to do with the shapes of these orbitals? Remember, the number of nodal surfaces within these orbitals is always equal to n-1. What kind of nodes are there in these orbitals? Answer: There are nodal planes and cones (aka: 'angular' nodes), and nodal spheres (aka: radial nodes). How do these influence the shapes of atomic orbitals? How does the most probable distance for the electron vary with different orbitals. You should be able to identify an orbital (1s, 2s, 2p, 3s, etc…) based purely on the number and types of nodal surfaces within it. Check out that Ohio State Chemistry link on our website to get a good idea of how these nodal surfaces give you a 3-dimensional orbital.
How to COUNT nodal surfaces
First, you need to LOOK at the shapes and get them in your head. Each orbital type (s, p, d, and f ) has a specific shape that is governed by the nodals surfaces for that set of quantum numbers.
- total nodal surfaces = n−1
- nodal planes and/or cones = ℓ
- nodal spheres (radial nodes) = n−ℓ−1
More questions to get straight. What’s the maximum number of electrons that will fit into ANY single orbital? Each orbital type (s, p, d, f . . . ) comes in sets. How many orbitals per set? What is the maximum number of electrons per set? What are the relative energies of the various orbitals for the hydrogen atom? for other atoms? On the exam, READ carefully and know when the question refers to a single orbital or when it refers to an entire set of orbitals.
Electron Configurations
You should be able to write (OK, pick out the answer from a list) out the electron configuration of any element on the periodic table. Yes, you WILL have your own copy of the periodic table to use which will be on the back of the Quest bubblesheet you get. You should also be able to write out electron configurations for various atoms. Remember those spots where there is an exception to the usual filling order (like with Cr and Cu when filling the 3d orbital set.
The Periodic Table
First off, this thing will really help you out with those electron configurations. Make sure you use it when 'memorizing' the Aufbau filling order see Figure 12.31 in your book. Second, you can really learn a lot about elements by studying the trends in the table. Remember that rows are called periods and columns are called families or groups.
Physical Property Trends
Know what the following are (definitions) AND what their trends are on the periodic table: atomic radii, ionization energies, and electron affinities. This is section 12.15. A quick reminder here though. For ionization Energy (aka: ionization potential) — you need to also know the difference in first, second, third, etc… what does first, second, and third ionization energies refer to?
Groups and Classifications
Check out Figure 12.39 in section 12.16. Know your group names and what being in a “group” means chemically. We will refer to the representative elements many times when bonding comes along. What are the representative elements? They are the “A” elements on the periodic table (or 1, 2, 13-18). What are the alkali metals and where are they on the periodic table? Where are the alkaline earth metals, the halogens, and the noble gases. Where are the metals, nonmetals, and metalloids? What are the d-transition metals? f-transition metals?
What Equations do you have to memorize?
I already mentioned this at the top of the review sheet but I'm putting it here again to hopefully stop that one student from emailing me about whether or not I'm going to include formulas. I will include formulas - more than you'll even need. So know the ones you need and use them. I'll also include all constants needed (and a few extra to boot). Oh yeah, quantum number definitions (rules) are NOT formulas and I won't be printing them on the exam.
Standard Disclaimer
Any mistakes on this review sheet are NOT intentional. You should crosscheck all stated information. You should double check your book too.