Kinetics Rate Equations
for a generic reaction: aA --> products
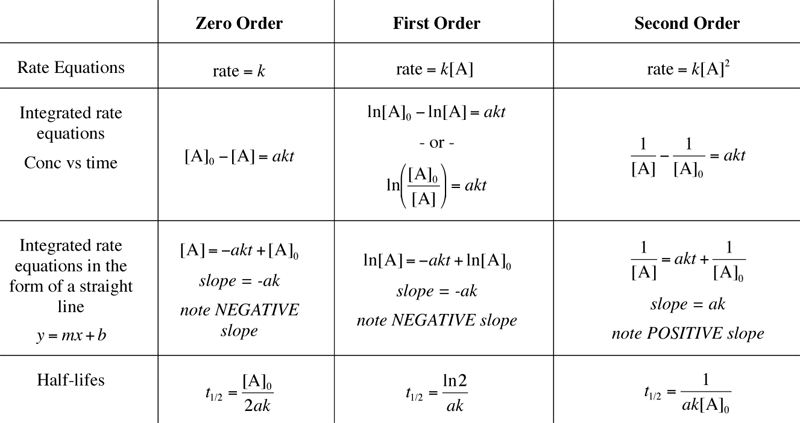
Some Things to NOTICE here
- note the similarities (and differcences) in the integrated rate laws
- all the same on the RIGHT side (akt)
- Each can be shown as the DIFFERENCE in two terms on the LEFT side
- The zero order concentration terms are PLAIN concentration.
- The 1st order concentration terms are NATURAL LOG terms.
- The 2nd order concentration terms are INVERSE terms.
- NOTE the Half-Life's dependence on concentration for EACH type.
- 0 order: DIRECTLY proportional to the initial concentration.
- 1st order: INDEPENDENT of any concentration!!!
- 2nd order: INVERSELY proportional to the initial concentration.
- The straight line plots all have slopes equal in magnitude to
ak, however, zero and first order have NEGATIVE slopes and second
order has a POSITIVE slope.
